Introduction
Type 1 diabetes mellitus (DM1) is a multifactorial and chronic endocrine disease characterized by losing of insulin-producing pancreatic β-cells, which occurs as a result of an autoimmune reaction by for–ming autoantibodies and autoreactive T-lymphocytes in these cells factors [8, 13, 15]. Patients with DM1 have an increased susceptibility to infections, especially viral ones, which is associated with the inhibition of the protective functions of the body as a result of immune system disorders, increased cell adhesion of microorganisms, the presence of micro- and macroangiopathy, neuropathy, and the development of other pathogenetic processes [7, 12, 16]. In the pathogenesis of DM1 and many of its complications, an important place belongs to the violation of the functional capacity of the immune system, which is the subject of numerous studies [4, 6]. Patients with DM1 have significant changes in chemotaxis, a decrease in the bactericidal activity of neutrophils, increased production of reactive oxygen species, leukotrienes, secretion of lysosomal enzymes, and changes in the level of intracellular calcium are often observed [2, 16].
Diseases of the ENT organs and respiratory tract are frequent reasons for seeking medical help. A special attention and methodological approach requires the treatment of DM1 in the case of its combination with other chronic diseases, for example, with chronic tonsillitis (ChT). ChT increases metabolic and functional disorders in the body, and also leads to decompensation of carbohydrate metabolism and even to ketoacidosis, which deteriorate the patholo–gical process in the tonsils [1, 3]. In conditions when the development and course of DM1 is complicated by the activation of foci of infection and often septic states, the evaluation of the functional activity of phagocytes, in which neutrophils dominate, may allow a more correct justification of the use of effe–rent therapy [1, 5].
Considering the effectiveness of immunomodulatory therapy for DМ1 and its complications, one of the most important problems is the search for new effective and harmless immunocorrecting agents with high pharmacological activity. Therefore, it was re–levant to study the effect of BNO 1030 Bionorica AG (Germany) on the functional and phenotypic characteristics of cells of tonsil in patients with chronic tonsillitis in vitro. Accordingly, one possible explanation for the abnormal leukocyte function in diabetes mellitus might be a down-regulation of adhesion molecules that regulate leukocyte recruitment during the course of inflammatory processes. The impaired local exudative cellular reaction in alloxan-induced diabetic rats is a consequence of defective leukocyte-endothelial interactions. Intravital microscopic examination of the internal spermatic fascia microcirculatory network showed that a reduced number of leukocytes rolling along the venular endothelium is observed from the early stages of diabetes. Considerable support to these clinical investigations was given by experimental studies on diabetic rats. Insulin restores the appropriate response to injury through a direct or indirect action on endothelial cells and leukocytes. For future studies the challenge remains to better understand the integration of adaptive immune systems with the endocrine system, providing new insights into how inflammation is regulated.
The purpose of this study was to evaluate the effect of the BNO 1030 on the phagocytic activity of blood leukocytes in rats with DM1.
Materials and methods
The studies were carried out on intact male Wistar rats weighing 130–150 g. The maintenance of animals and conducting experiments with them were carried out in accordance with generally accepted international requirements for work with experimental animals and in accordance with the relevant national provisions for conducting experimental work [4]. The experimental animals were kept on a standard ration of the vivarium, with free access to food and water. Experimental DM1 in rats was induced by a single intraperitoneal injection of streptozotocin (Sigma-Aldrich Co. LLC, USA) with a dose of 55 mg/kg body weight, diluted in 0.1 M citrate buffer, pH 4.5 before administration to animals. The rats of the control group of the same age were intraperitoneally injected with 0.5 ml 0.1 M citrate buffer, pH 4.5. Blood sampling in animals was performed in the morning after fasting (12 hours) from the retrobulbar venous sinus of the eye, under light ether anesthesia. The blood glucose level was determined using a Precision Xtra Plus blood glucose meter (MediSense UK Ltd., UK). Leukocytes were obtained on the day of the experiment from peripheral blood of experimental animals by osmotic shock of erythrocyte membranes. To this end, the heparinized blood was mixed with a cold lysis solution (0.15 mol/L NH4Cl, 1 mmol/L KHCO3, 0.1 mmol/L EDTA, pH 7.2–7.4) in a ratio of 1 : 20 and after a thorough shaking Incubated for 10 minutes at 37 °C. After the lysis time of the erythrocytes, the samples were centrifuged (400 g, 5 min) for the precipitation of leukocytes. The supernatant was removed and the pellet was washed twice with saline by centrifugation (400 g, 5 min). The washed pellet was resuspended at a concentration of 2 × 106 cells/ml in phosphate buffered saline (PBS) (Phosphate buffered saline, pH 7.2). Blood serum was obtained by centrifugation of whole blood in a centrifuge Eppendorf 5810R (USA) at 1300 g for 7 min at 22 °C. Serum was stored at –72 °C until use.
Evaluation of redistribution between different leukocyte populations was carried out using two parameters of the COULTER EPICS XL protocol cytofluorimeter (Beckman Coulter, USA) equipped with an argon laser (λ = 488 nm): direct (FS, cell size) and lateral light scattering (SS, granularity Cells), Fig. 1.
The percentage of phagocytic cells and macrophage absorption by live fluorescent bacteria in the samples were determined using fluorescent live bacteria Escherichia coli, according to the method of [9]. The cells were incubated for 1 hour at 37 °C with live
fluorescent bacteria of Escherichia coli at a concentration of 6 × 106 bacteria/ml in 1% BSA/PBS buffer. After washing in 1% BSA/PBS buffer, an analysis was made of the percentage of phagocytic cells in the flow cytofluorimeter, Fig. 2.
The principle of the method for determining phagocytic activity is based on the absorption of E.coli by phytochemicals, monocytes and macrophages (capable of expressing Green Fluorescent Protein (GFP) with a molecular weight of 26.9 kDa by the phagocytosis). This protein fluoresces with green light when excited by light blue (laser, 488 nm). Accordingly, the more bacteria are absorbed by the cell, the higher the fluorescence intensity of GFP in it.
The statistical processing of the obtained results was carried out using the Statistica 6.0 program. Samples were compared using Student’s t-test. The results are presented as the mean value (M) and the standard error of the mean value (± m). The difference was considered statistically significant at p < 0.05.
Results
DM1 occurs as a result of autoimmune inflammation and caused by various exogenous degradation factors of β-cells of the pancreas, accompanied by numerous complications due to relative and/or absolute insufficiency of insulin [15, 19]. A characteristic feature of the development of DM1 is a disruption of the metabolism of carbohydrates, which results in an increase in blood glucose concentration. Therefore, to validate the experimental model of DM1, it was important to determine the level of glucose in the blood of the animals. At the beginning of the experiments, blood glucose levels were almost the same in all the studied groups, but after six weeks of DM-1development, glucose levels in the blood increased 2.3-fold compared to control animals, which is a confirmation of the development of uncompensated hyperglycemia in animals.
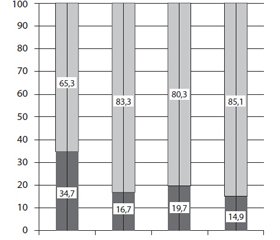
As evidenced by the data obtained, shown in Fig. 3, changes in the redistribution of leukocytes were detected. Thus, the amount of agranulocytes in the blood of diabetic rats increased by 15 % compared with the control animals, while the number of granu–locytes, on the contrary, decreased. The revealed changes in their redistribution indicate the reaction of the hematopoietic apparatus to the development of pathological processes in the organism of animals induced by diabetes mellitus, which is consistent with the literature data, which indicate that changes in the distribution of the main types occur at various blood diseases and the appearance of other pathological conditions [10, 14].
When Imuprеt was administered to both control and diabetic rats, an increase in the amount of agranu–locytes was also observed. Since the components of the agglutination are lymphocytes and monocytes, the data obtained may indicate modulating the effect of the drug on the immune system.
For a more complete evaluation of phagocytic activity, the phagocytic number (the average number of microbes that are absorbed by one phagocyte) was determined. The obtained data indicate that the phagocyte count in the group of rats with DM1 significantly decreases, namely, less than 42 % compared to the control animals, Fig. 4. When administered to control rats of the drug BNO 1030, no reliable chan–ges in the magnitude of the phagocytic number were detected. However, in the group of diabetic rats receiving BNO 1030, an increase in phagocytosis was found to be 24 % compared to a group of diabetic animals that did not receive the drug.
It was observed both in diabetes and in the admi–nistration of the drug to experimental animals in comparison with the control group evaluating the phagocytic index (the ratio of phagocytic cells to the total number of cells in leukocytes) (Fig. 5). It should be noted that the observed decrease in the phagocytic index correlates with the total content of granulocytes in the samples (Fig. 3), taking into account the redistribution between the two major types of leukocytes in the blood at the experimental DM1. The decrease of the investigated parameters of activity of phagocytosis testifies to violations in the system of non-specific cellular immunity. This can be the result of reduced production of phagocytes, their rapid decay, violation of their mobility, violation of absorption and neutra–lization of foreign agents, and the like.
Discussion
According to the obtained data, it becomes appa–rent that hyperglycemia leads to a decrease in the acti–vity of the immune system, which agrees with the results of other authors who found that patients with diabetes form a risk group for susceptibility to infections as a result of reduced immunity [8, 11].
Studies with diabetic rats and also showed a decreased neutrophil migration, phagocytosis capacity and hydrogen peroxide production. Furthermore, the reduction of blood glucose levels by insulin treatment of diabetic patients or rats has been reported to be significantly correlated with improvement of neutrophil phagocytosis capacity. During an inflammatory response, leukocytes roll along the lining endothelium of post-capillary venules and eventually become firmly attached to the vascular wall before migrating into tissues. Specific adhesion glycoproteins expressed on the surface of leukocytes and endothelial cells play a re–levant role in the accumulation of leukocytes in the inflammatory lesion. Members of the selectin family of cell adhesion molecules are thought to mediate leukocyte rolling along the walls of the microvasculature.
Another important advance in understanding the pathogenesis of neutrophil dysfunction and inflammatory disorders in diabetes is the observation that glucose or its analogues interact with proteins or lipids. The end products of this non-enzymatically catalyzed reaction, termed advanced glycation (glycosylation or glycoxidation) end products (AGEs), have been linked to the development of long-term complications of diabetes. Early glycation and oxidation processes result in the formation of reversible Schiff bases, which undergo an intramolecular rearrangement to form the Amadori products like glycated hemoglobin (HbA1c) that is elevated in diabetic patients. A small proportion of these products undergo further slow and irreversible chemical rearrangements to form AGEs, which accumulate in the vasculature under conditions that are accelerated during hyperglycemia and when protein turnover is delayed.
Conclusions
Thus, the data obtained indicate a pronounced reduction in the body’s resistance to various infections, especially when they occur on the background of DM1.
A confirmation of this is the process of phagocytosis, which undergoes significant changes in patients with DM1.
These changes, in the light of experimental data, partially prevent the use of the drug BNO 1030, which is able to exhibit immunomodulatory effect in patients with DM1.
1. It was established that in the experimental DM1 changes in the redistribution of the main types of leukocytes occur. When BNO 1030 was administered to control and diabetic animal, the amount of agranulocytes increased, which may indicate a modulating effect of the drug on the immune system.
2. The phagocyte count in the group of DM1 patients was significantly lower and decreased by 42 % compared with the control animals. When administered to diabetic rats, BNO 1030 showed an increase in phagocyte count by 24 % compared to a group of diabetic animals that did not receive the drug.
3. The reduction of the phagocytic index in both groups indicates a violation of the system of nonspecific cellular immunity
Conflicts of interests. Author declares the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Список литературы
1. Гавриленко Ю.В. Особенности поражения ЛОР-органов у детей и подростков с сахарным диабетом 1 типа // Современная педиатрия. — 2015. — № 7(71). — С. 62-65. doi: 10.15574/SP.2015.71.62.
2. Лайко А.А., Гавриленко Ю.В. Характер ураження ЛОР-органів у дітей, хворих на цукровий діабет 1 типу // Ринологія. — 2014. — № 1. — С. 61-65.
3. Перцева Н.О. Проявления инсулинорезистентности у пациентов, длительно страдающих сахарным диабетом 1-го типа, пути ее коррекции / Перцева Н.О., Марциник Е.Н., Чупсинова Т.В. // Международный эндокринологический журнал. — 2017. — Т. 13(1). — С. 23-27.
4. Adeghate E. An update on the etiology and epidemiology of diabetes mellitus / E. Adeghate, P. Schattner, E. Dunn // Ann. NY Acad. Sci. — 2006. — Vol. 1084. — P. 1-29.
5. Bicker H., Höflich C., Wolk K. et al. A Simple assay to measure phagocytosis of live bacteria // Clinical Chemi–stry. — 2008. — Vol. 54(5). — P. 911-915. doi: 10.1373/clinchem.2007.101337.
6. Casqueiro J., Casqueiro J., Alves C. Cresio Infections in patients with diabetes mellitus: A review of pathogenesis // Indian J. Endocrinol. Metab. — 2012. — Vol. 16(1). — P. 27. doi: 10.4103/2230-8210.94253.
7. Chattopadhyay S. Animal models in experimental diabetes mellitus / S. Chattopadhyay, M. Ramanathan, J. Das, S. K. Bhattacharya // Indian Journal of Experimental Biology. — 1997. — Vol. 35(11). — P. 1141-1145.
8. Bilgic S., Aktas E., Salman F. et al. Intracytoplasmic cytokine levels and neutrophil functions in early clinical stage of type 1 diabetes // Diabetes Res Clin. Pract. — 2008. — Vol.79(1). — P. 31-36.
9. Valle A., Giamporcaro G.M., Scavini M. et al. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes // Diabetes. — 2013. — Vol. 62(6). — P. 2072-2077. doi: 10.2337/db12-1345.
10. Knip M., Veijola R., Virtanen S.M. et al. Environmental triggers and determinants of type 1 diabetes // Diabetes. — 2005. — Vol. 54. — P. 125-136.
11. James S., Gallagher R., Dunbabin J., Perry L. Prevalence of vascular complications and factors predictive of their development in young adults with type 1 diabetes: systematic literature review // BMC Res Notes. — 2014. — Vol. 7. — P. 593-603. doi: 10.1186/1756-0500-7-593.
12. Zhang C., Yang J., Jennings L.K. Leukocyte-derived myeloperoxidase amplifies high-glucose-induced endothelial dysfunction through interaction with high-glucose-stimulated, vascular non-leukocyte-derived reactive oxygen species // Diabetes. — 2004. — Vol. 53(11). — P. 2950-2959.
13. Szablewski L., Sulima A. The structural and functional changes of blood cells and molecular components in diabetes mellitus // Biol. Chem. — 2016. — Vol. 3. — P. 66-70.
14. Popov D. Endothelial cell dysfunction in hyperglycemia: Phenotypic change, intracellular signaling modification, ultrastructural alteration, and potential clinical outcomes // International Journal of Diabetes Mellitus. — 2010. — Vol. 3. — P. 189-195.
15. Molteni R., Fabbri M., Bender J.R., Pardi R. Pathophysiology of leukocyte-tissue interactions // Curr. Opin. Cell Biol. — 2006. — Vol.18(5). — P. 491-498.
16. Bertoni A.G., Saydah S., Brancati F.L. Diabetes and the risk of infection-related mortality in the U.S. // Diabetes Care. — 2001. — Vol. 24(6). — P. 1044-1049.

/90-1.jpg )
/91-1.jpg )
/91-2.jpg )
/92-1.jpg )