Резюме
Мета: проаналізувати структурні зміни слизової оболонки шлунка та оцінити роль системи оксиду азоту в цих процесах у пацієнтів iз хронічним гастритом та кишковою метаплазією. Матеріали та методи. Морфологічне дослідження проводилося в 38 пацієнтів iз хронічним гастритом та атрофією, локальною (антральний відділ шлунка) або дифузною кишковою метаплазією. Концентрація оксиду азоту була визначена у вмісті шлунка та в крові всіх пацієнтів. Результати. Морфометричне дослідження показало помітне зниження (до (0,12 ± 0,04) %) ядерно-цитоплазматичного співвідношення в пацієнтів iз кишковою метаплазією порівняно з тими, хто мав атрофію. Еліптичність ядер клітин також зменшувалася з розвитком кишкової метаплазії та в пацієнтів без метапластичних змін дорівнювала (0,76 ± 0,04) %, у випадках локальної кишкової метаплазії — (0,65 ± 0,11) %, при дифузнiй метаплазії — (0,41 ± 0,12) %. Встановлено, що у хворих iз кишковою метаплазією, локалiзованою в антральному відділі, рівень оксиду азоту в шлунковому соку збільшується і на 24–65 % перевищує контрольну величину. Розглянуто можливий механізм загибелі клітин слизової оболонки шлунка внаслiдок апоптозу та некрозу в умовах гіперпродукції оксиду азоту. Висновки. Iнгібуючий ефект оксиду азоту на шлункову секрецію реалізується за рахунок його стимулюючого впливу на активність циклооксигенази та синтезу простагландинів.
Цель: проанализировать структурные изменения слизистой оболочки желудка и оценить роль системы оксида азота в этих процессах у пациентов с хроническим гастритом и кишечной метаплазией. Материалы и методы. Морфологическое исследование проводилось у 38 пациентов с хроническим гастритом и атрофией, локальной (антральный отдел желудка) или диффузной кишечной метаплазией. Концентрация оксида азота была определена в содержимом желудка и в крови всех пациентов. Результаты. Морфометрическое исследование показало заметное снижение (до (0,12 ± 0,04) %) ядерно-цитоплазматического соотношения у пациентов с кишечной метаплазией по сравнению с теми, у кого отмечалась атрофия. Эллиптичность ядер клеток также уменьшалась с развитием кишечной метаплазии и у пациентов без метапластических изменений составляла (0,76 ± 0,04) %, в случаях локальной кишечной метаплазии — (0,65 ± 0,11) %, при диффузной метаплазии — (0,41 ± 0,12) %. Установлено, что у больных с локальной кишечной метаплазией, локализованной в антральном отделе желудка, уровень оксида азота в желудочном соке увеличивается и на 24–65 % превышает контрольные показатели. Рассмотрен возможный механизм гибели клеток слизистой оболочки желудка вследствие апоптоза и некроза в условиях гиперпродукции оксида азота. Выводы. Ингибирующий эффект оксида азота на желудочную секрецию реализуется за счет его стимулирующего влияния на активность циклооксигеназы и синтеза простагландинов.
Background. The purpose was to analyze structural changes in gastric mucosa and to assess the role of nitric oxide system in these processes in patients with chronic gastritis and intestinal metaplasia. Materials and methods. Morphological study was conducted in 38 patients with chronic gastritis with atrophy, intestinal metaplasia located in the antrum and diffuse intestinal metaplasia. Concentration of nitric oxide was measured in gastric contents and in the blood of all patients. Results. Morphometric study showed a marked decrease (to (0.12 ± 0.04) %) in the nuclear-cytoplasmic ratio in patients with intestinal metaplasia compared to patients with atrophy, while the ellipticity of the native cell nuclei also decreased with the development of intestinal metaplasia, and in patients without metaplastic changes was equal to (0.76 ± 0.04) %, for cases of antrum-localized intestinal metaplasia — (0.65 ± 0.11) %, and for the diffuse metaplasia — (0.41 ± 0.12) %. It was found that in patients with antrum-localized intestinal metaplasia, the level of nitric oxide in gastric juice increased and was 24–65 % higher than the control values. A possible mechanism of gastric mucosa cell death with apoptosis and necrosis under hyperproduction of nitric oxide is considered. Conclusions. The inhibitory effect of nitric oxide on acidic gastric secretion is realized at the expense of its stimulating effect on the activity of cyclooxygenase and the synthesis of prostaglandins.
Introduction
Chronic gastritis — the presence of aggressive inflammation in the gastric mucosa (GM), which often results in its destruction, is one of the most common pathologies affecting upper digestive system. Progressive specific changes in the GM, both functional (acid-forming disorder) and structural (atrophy), are the highest independent risks for gastric cancer known so far [1, 3].
According to the modern international scientific position, atrophy is defined as “loss of the corresponding gland structure”. This definition covers both the “loss” of the natural glands (fibrosis) and the metaplastic changes of the corresponding glands, as a result of which they acquire a characte-ristic structure and lose the ability to perform their function [1].
All the changes that occur in chronic gastritis include cell death (from the structural breakdown point of view) and the development of oxidative stress [2, 3]. Nitric oxide (NO) is a membrane-permeable gaseous secondary messenger that participates in signal transduction of the central and autonomic nervous systems and regulates many respiratory, genitourinary and gastrointestinal functions [4–7].
In the human stomach, NO and a number of other bioactive nitrogen oxides are formed from precursors that come with food and saliva [8–10, 16]. Hydrochloric acid secreted by the parietal cells of the gastric mucosa is a key catalyst for many reactions involving nitric oxide and its derivatives. The key link here is the protonation of nitrites in the lumen of the stomach and the formation of nitric acid. This acid itself is essentially a nitrosating agent, but can also spontaneously disintegrate with the formation of NO and other nitrogen oxides [11, 12]. In the stomach, due to the transformation of nitrates and nitrites, nitrosation products are formed, which can be transported to the blood and act as a powerful vasodilator, capable of lowering arterial pressure [13, 18].
Factors that affect the production of NO and other reactive nitrogen oxides in the stomach include pH, the amount of nitrites, the presence of vitamin C, thiocyanate, polyphenols, the availability of proteins, thiols, and oxygen pressure. The formation of NO in the stomach is also an acid-dependent process, which can be suppressed by proton pump inhibitors that increase the pH of the stomach [19].
The biological significance of NO formation and its effect on preserving the integrity of GM remains not yet fully understood, but its role in the regulation of the periodic motor activity of the stomach is realized through diffusion signal transmission [20]; NO, as a secondary mediator, provides vasodilator effects of the vagus nerve, acts as a neurotransmitter in the peripheral nerves innervating the gastrointestinal tract [20, 21]. However, despite the predominantly positive role of NO, the amount of nitric oxide released in the stomach is critically important. The negative effect of NO begins to occur when its concentration drops sharply or increases, which leads to functional and structural damage to the organ [20].
It should be noted that the literature data on the study of NO functional activity are based mostly on the results of studying the final products of metabolism (nitrates and nitrites) in the blood serum. Meanwhile, these data may depend on a number of factors, in particular, the presence of concomitant pathology of other organs and body systems. The study of the NO levels in gastric juice in diseases of the upper gastrointestinal tract, as a marker for assessing the activity of destructive processes in the GM, is of considerable interest.
The purpose of this study was to analyze structural changes in gastric mucosa and to evaluate the role of nitric oxide system in these processes in patients with chronic gastritis and intestinal metaplasia.
Materials and methods
The present study was undertaken in order to analyze the morphological and biochemical changes in 38 patients with chronic gastritis divided into three groups: group I — atrophy of the GM (n = 6); group ІІ — intestinal metaplasia located in the antrum (n = 12); group III — diffuse intestinal metaplasia (n = 20). The control group consisted of appa–rently healthy persons.
Structural changes were analyzed in GM biopsies obtained during gastroscopy (2 biopsies from the fundus and antrum of the stomach on lesser and greater curvatures, and 1 biopsy from the incisura angularis). Imprints for bacteriological examination for the presence of helicobacter infection were made in all patients, and in the presence of helicobacter in the gastric mucosa, the degree of its severity was determined.
For histological investigations, gastric biopsies were immediately fixed in 10% formalin, paraffin-embedded sections were cut at 3–5 microns and mounted on glass slides. Sections were deparaffinized and stained with hematoxyli–neosin or periodic acid Schiff (PAS) reaction was performed, morphometric studies were carried out with a correlation analysis of the data.
For the morphometric study, histological specimens were photographed using 1000× light microscope XSP-139TP and measured with ImageJ 1.45 software (National Institutes of Health, USA).
The concentration of NO in the gastric contents was determined by the method of Reinhold and Wilson.
The analysis of the results was performed using the me–thods of descriptive statistics: the calculation of the mean, 95% interval and the standard error in the sample, the result was recorded as M ± m. Comparison of the mean values was performed using parametric methods. To determine the relationship between the two indicators, a correlation analysis was conducted to determine the reliable Pearson correlation coefficients and Spearman rank coefficients.
The comparative analysis was conducted with the indicators in the control group consisted of apparently healthy persons. The evaluation of the studied indicators was given in accordance with their changes: NO in gastric juice — (14.32 ± 19.48) μmol/l; NO in the blood — (25.0 ± 58.3) μmol/l; cholic acid in gastric juice — (0.000 ± 0.006) mmol/l.
Results and discussion
In order to improve the accuracy of the histological study, the following morphometric indices were calculated on the microphotographs obtained with the help of 1000× imaging lens: nuclear-cytoplasmic ratio (NCR), nuclear ellipticity coefficient (NEC) and the nuclear homogeneity coefficient. Given the structural difference between the native cells of the stomach and intestinal glands, these indices were calculated separately for metaplastic cells and for native cells in the zones bordering those affected by intestinal metaplasia.
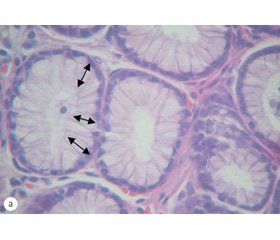
Changes in the NCR are among the first and most precise signs of intestinal metaplasia and dysplasia in GM. Thus, if NCR of unchanged gastric glands was equal to (0.18 ± 0.02) %, in cells affected by intestinal metaplasia it was significantly increased — to (0.33 ± 0.03) % (p < 0.05). It is important to note that the increase of NCR in metaplastic cells was most often accompanied by its decrease in adjacent areas. This difference was particularly noticeable in the biopsy samples of patients from group III. In this group, according to the histological study, hyperplasia of the GM glands was detected in 42.9 % of cases. A morphometric study showed a significant reduction in NCR to (0.12 ± 0.04) % (Fig. 1), with a slight decrease in NCR even in cases where no marked hyperplastic changes were noted in the histological and endoscopic examination.
Another sign of the initial changes in the native structure of the GM is NEC. The accuracy of NEC is slightly higher than that of the NCR, but does not depend on the overall size of the cells, which reduces the margin of error from the orientation of the sections. In our study, the NEC of native cells decreased with the development of IM. Thereby, for group I, it was equal to (0.76 ± 0.04) %, for group ІІ — (0.65 ± 0.11) %, and for group ІІІ — (0.41 ± 0.12) % (Fig. 2). Statistical analysis revealed a strong correlation between this parameter and NCR (r = 0.55, p ≤ 0.05). The NEC of metaplastic cells ranged from (0.66 ± 0.01) % to (0.89 ± 0.09) %, regardless of the study group.
Pleomorphism of the nuclei, which is the hallmark of more severe structural changes in the GM, is characterized by significant fluctuations in the mean diameter of the epithelial cell nucleus and the increase in NCR.
In our study, there were no significant differences between the values of NCR in the analyzed groups (Fig. 3a). However, in one case, against the background of intestinal metaplasia, which was characterized by a large area of diffusion (> 75 % of the total biopsy area), but a reduced number of goblet cells, a significant increase in NCR was noted (Fig. 3b) that allowed considering this case as dysplasia of the GM.
NO, the production of which may increase at the expense of Helicobacter life-sustaining activity or decrease due to the development of oxidative stress accompanying IM [3], has the ability to upregulate the enzymatic activity of DNA methyltransferase without affecting the expression level of mRNA [3]. In our study, the content of NO in gastric juice tended to increase only in the second group of patients, by 14 % compared to the controls (Table 1).
Statistical data processing allowed us to detect a weak inverse correlation between the area of intestinal metaplasia and the content of NO in the blood (r = 0.355, p = 0.037), but we believe that this link is more likely mediated through the factors of chronic inflammation, signals from macrophages, interleukin 1 or tumor necrosis factor α, which is also confirmed by the lack of areliable association with the NO content in gastric juice.
On the other hand, nitric oxide may be an aggressive factor due to its ability to inhibit cyclooxygenase under certain conditions, to increase the cytotoxicity of hydrogen peroxide, and to initiate apoptosis. NO reacts with cellular iron-containing proteins (such as aconitase, complex I–III mitochondrial electron transfer chains) and completely inactivates them by S-nitrosylation. In the presence of oxygen, active NO intermediates (including peroxynitrite), which directly manifest cytotoxic action, are formed [14, 15, 17].
Hyperproduction of nitric oxide also causes inhibition of glyceraldehyde-3-phosphate dehydrogenase by ribosylation and nitrosylation. This leads to inhibition of glycolysis and, subsequently, to a disturbance of energy metabolism. All of these undesirable effects are mediated by the activation of NO synthase and excessive NO production. In high concentrations, nitric oxide is a factor in endogenous intoxication, which determines its cytotoxic effect and causes the death of cells and tissues through the mechanisms of apoptosis and necrosis [12].
Conclusions
Morphometric study showed a significant decrease (to (0.12 ± 0.04) %) in the nuclear-cytoplasmic ratio of patients with intestinal metaplasia compared to patients with atrophy, while the ellipticity of the nuclei of the native cells also decreased with the development of intestinal metaplasia, and in patients without metaplastic changes was equal to (0.76 ± 0.04) %, for cases of antrum-localized intestinal metaplasia — (0.65 ± 0.11) %, and for the diffuse metaplasia — (0.41 ± 0.12) %. It was found that in patients with antrum-localized intestinal metaplasia, the level of nitric oxide in gastric juice increased and was 24–65 % higher than the control values. It is assumed that the inhibitory effect of nitric oxide on acidic gastric secretion is realized at the expense of the stimulating effect of nitric oxide on the activity of cyclooxygenase and the synthesis of prostaglandins. A possible mechanism of gastric mucosa cell death due to apoptosis and necrosis under conditions of hyperproduction of nitric oxide is considered.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Список литературы
1. Clinicopathological features of gastric adenocarcinoma of the fundic gland (chief cell predominant type) by retrospective and prospective analyses of endoscopic findings / T. Chiba, K. Kato, T. Masuda [et al.] // Dig. Endosc. — 2016. — Vol. 28(7). — P. 722-730.
2. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases / A. Bhattacharyya, R. Chattopadhyay, S. Mitra [et al.] // Physiological Reviews. — 2014. — Vol. 94(2). — P. 329-354.
3. Helicobacter pylori-induced gastric inflammation and gastric cancer / Fei Wang, Wenbo Meng, Bingyuan Wang // Cancer Letters. — 2014. — Vol. 45(2). — P. 196-202.
4. Проблема оксиду азоту в неврології: Монографія / В.О. Малахов, Г.М. Завгородня, В.С. Личко [та ін.]. — Суми: Видавництво СумДПУ ім. А.С. Макаренка, 2009. — 242 с.
5. Маеда Х. Оксид азота и кислородные радикалы при инфекции, воспалении и раке / Х. Маеда, Т. Акаике // Биохимия. — 1998. — № 63(7). — С. 1007-1019.
6. Lanas A. Role of nitric oxide in the gastrointestinal tract [Електронний ресурс] / A. Lanas // Arthritis Res. Ther. — 2008. — Vol. 10 (Suppl. 2). — Режим доступу: http://arthritisresearch.com/supplements/10/S2/S4
7. Role of nitric oxide in physiology and pathology of the gastrointestinal tract / A. Stanek, K. Gavron, A. Gadowska-Cicha [et al.]. // Mini Reviews in Medicinal Chemistry. — 2009. — Vol. 8(14). — P. 1549-60.
8. Максимович Я. Оксид азоту в патогенезі виразки шлунка / Я. Максимович, О. Дробінська, Ю. Гавриленко // Вісн. Київ. нац. ун-ту ім. Т. Шевченка. — 2007. — № 49–50. — С. 73-76.
9. Lajoix A.D. RNA silencing targeting PIN (protein inhibitor of neuronal nitric oxide synthase) attenuates the development of hypertension in young spontaneously hypertensive rats / S.C. Wang, K.M. Lin, S.J. Chien [et al.] // J. Am. Soc. Hypertens. — 2014. — Vol. 8(1). — P. 5-13.
10. Nitric oxide and the gastrointestinal tract / N.I. Kochar, A.V. Chandewal, R.L. Bakal, P.N. Kochar // International Journal of Pharmacology. — 2011. — Vol. 7. — P. 31-39.
11. Role of nitric oxide in physiology and pathology of the gastrointestinal tract / A. Stanek, A. Gadowska-Cicha, K. Gawron [et al.] // Mini-Reviews in Medicinal Chemistry. — 2008. — Vol. 8(14). — P. 1-12.
11. Shah V. Nitric oxide in gastrointestinal health and disease / V. Shah, G. Lyford, G. Gores [et al.] // Gastroenterology. — 2004. — Vol. 126. — P. 903-913.
12. Dietary nitrite in nitric oxide biology: a redox interplay with implications for pathophysiology and therapeutics / B.S. Rocha, B. Gago, C. Pereira [et al.] // Curr. Drug Targets. — 2011. — Vol. 12. — P. 1351-1363.
13. Ethyl nitrite is produced in the human stomach from dietary nitrate and ethanol, releasing nitric oxide at physiological pH: potential impact on gastric motility / B.S. Rocha, B. Gago, R.M. Barbosa [et al.] // Free Radic. Biol. Med. — 2015. — Vol. 45. — P. 404-412.
14. Клекот О.О. Метаболізм нітрогену оксиду при СЧВ-асоційованій легеневій артеріальній гіпертензії / О.О. Клекот // Укр. ревматол. журн. — 2011. — № 82. — С. 160-166.
15. Gilchrist M. Inorganic nitrate: marker or mediator of mortality? / M. Gilchrist, A. Shore // J. Am. Heart Assoc. — 2017. — Vol. 6(11). — P. e007782.
16. Особливості періодичної активності шлунка за умов дисбалансу NO-ергічної системи / О.В. Севериновська, О.О. Галінський, А.І. Руденко [та ін.] // Вісн. Дніпропетр. ун-ту. Біологія. Mедицина. — 2014. — № 5(1). — C. 71-78.
17. Toda N. Gastrointestinal function regulation by nitrergic efferent nerves / N. Toda, A.G. Herman // Pharmacol. Rev. — 2005. — Vol. 57(3). — Р. 315-338.
18. Активність синтази оксиду азоту та вміст пероксинітриту у клітинах слизової оболонки шлунка щурів за умов експериментальної стресової виразки / Я.С. Максимович, М.В. Миленко, О.В. Дробінська [та ін.] // Вісн. Дніпропетр. ун-ту. Біологія. Eкологія. — 2009. — № 17(1). — С. 134-142.
19. Ma L. Endothelial nitric oxide synthase modulates gastric ulcer healing in rats / L. Ma, J.L. Wallace // Am. J. Physiol. Gastrointest. Liver Physiol. — 2005. — Vol. 279. — P. 341-346.
20. Nitric oxide donor protects against acetic acid-induced gastric ulcer in rats via S-nitrosylation of TRPV1 on vagus nerve / Ting Han, Yan Tang, Jing Li [et al.] // Scientific Reports. — 2017. — Vol. 7. — Р. 2063.
21. Участь системи синтази оксиду азоту в розвитку та відновленні стрес-індукованих уражень слизової оболонки шлунка / О. Богданова, Л. Кузьменко, О. Дробінська, Л. Остапченко // Вісн. Київ. нац. ун-ту ім. Т. Шевченка. — 2007. — № 12. — С. 5-8.

/11-1.jpg)
/12-1.jpg)