Международный эндокринологический журнал Том 15, №2, 2019
Вернуться к номеру
Роль розладів функціонального стану ендотелію в розвитку і прогресуванні діабетичної периферичної симетричної полінейропатії залежно від генетичних чинників
Авторы: Zoriy I.A., Pashkovska N.V.
Bukovinian State Medical University, Chernivtsi, Ukraine
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
Мета. Оцінка показників функціонального статусу ендотелію у хворих на діабетичну периферичну симетричну полінейропатію (ДПСП) внаслідок цукрового діабету (ЦД) 2го типу залежно від поліморфізму ендотеліального гена NOсинтази (eNOS). Матеріали та методи. Під спостереженням перебувало 110 хворих на ДПСП на тлі ЦД 2го типу, які сформували основну групу. Середній вік пацієнтів становив 54,2 року (від 38 до 72 років). Розподіл пацієнтів здійснювався за тяжкістю ДПСП: першу групу становили 32 (29,1 %) пацієнти з початковими проявами ДПСП; друга група включала 58 (52,7 %) пацієнтів із помірним ступенем; третя група — 20 (18,2 %) пацієнтів із вираженим ступенем ДПСП. Усім пацієнтам проведено неврологічний огляд за шкалою нейропатичного симптоматичного і нейропатичного функціонального підрахунку. Функціональний стан ендотелію досліджували за вмістом стійких метаболітів оксиду азоту (NO) в крові й числом циркулюючих ендотеліальних клітин у крові. Поліморфізм G894T в гені eNOS виявлено за допомогою полімеразної ланцюгової реакції. Результати. Згідно з отриманими даними, у більшості пацієнтів (78,5 %) відзначалося зменшення кількості NO2–NO3–. Число десквамованих ендотеліальних клітин було вірогідно збільшене в усіх досліджених пацієнтів порівняно з контрольною групою. При порівняльній оцінці ендотеліальних функціональних параметрів залежно від поліморфізму G894T в гені eNOS встановлено, що у хворих із гомозиготним генотипом для рідкісної T алелі спостерігалося вірогідне зниження вмісту NO2–NO3– — у 43,6 % (p < 0,05) порівняно з контрольною групою. Висновки. Встановлені вірогідні порушення функції ендотелію у хворих із помірним і вираженим ступенем діабетичної периферичної симетричної полінейропатії на тлі ЦД 2го типу. Результати дослідження підтвердили ймовірний вплив поліморфізму G894T в гені eNOS на утворення фенотипу, що обумовлює схильнiсть до розвитку мікроангіопатій.
Цель. Оценка показателей функционального статуса эндотелия у больных диабетической периферической симметричной полинейропатией (ДПСП) вследствие сахарного диабета (СД) 2го типа в зависимости от полиморфизма эндотелиального гена NOсинтазы (eNOS). Материалы и методы. Под наблюдением находились 110 больных ДПСП на фоне СД 2го типа, которые сформировали основную группу. Средний возраст пациентов составлял 54,2 года (от 38 до 72 лет). Распределение пациентов осуществлялось по тяжести ДПСП: первая группа состояла из 32 (29,1 %) пациентов с начальными проявлениями ДПСП; вторая группа включала 58 (52,7 %) пациентов с умеренной степенью; третья группа — 20 (18,2 %) пациентов с выраженной степенью ДПСП. Всем пациентам проведен неврологический осмотр по шкале нейропатического симптоматического и нейропатического функционального счета. Функциональное состояние эндотелия исследовали по содержанию стойких метаболитов оксида азота (NO) в крови и числу циркулирующих эндотелиальных клеток в крови. Полиморфизм G894T в гене eNOS выявляли с помощью полимеразной цепной реакции. Результаты. Согласно полученным данным, у большинства пациентов (78,5 %) отмечалось уменьшение количества NO2–NO3–. Число десквамированных эндотелиальных клеток было достоверно увеличено у всех исследованных пациентов в сравнении с контрольной группой. При сравнительной оценке эндотелиальных функциональных параметров в зависимости от полиморфизма G894T в гене eNOS установлено, что у больных с гомозиготным генотипом для редкой T аллели наблюдалось достоверное снижение содержания NO2–NO3– — у 43,6 % (p < 0,05) в сравнении с контрольной группой. Выводы. Установлены достоверные нарушения функции эндотелия у больных с умеренной и выраженной степенью диабетической периферической симметричной полинейропатии на фоне СД 2го типа. Результаты исследования подтвердили вероятное влияние полиморфизма G894T в гене eNOS на образование фенотипа, обусловливающего склонность к развитию микроангиопатий.
Background. The purpose was to examine indicators of endothelial functional status in patients with diabetic distal symmetric polyneuropathy (DDSP) on the background of type 2 diabetes mellitus (DM), which depends on the polymorphism of the endothelial nitric oxide synthase gene (eNOS). Materials and methods. One-hundred and ten patients with DDSP on the background of type 2 DM were examined and composed the basic group. The average age of patients was 54.2 years (from 38 to 72 years). The patients were distributed according to DDSP severity: the first group consisted of 32 (29.1 %) patients with the initial manifestations of DDSP; the second group included 58 (52.7 %) patients with moderate degree; the third group consisted of 20 (18.2 %) patients with severe DDSP. All patients underwent neurological examination using the scale of neuropathic symptomatic and neuropathic functional counting. The endothelial function was investigated by the content of stable metabolites of NO in the blood and the number of circulating endothelial cells in the blood. G894T polymorphism in eNOS gene was detected by polymerase chain reaction. Results. According to the data, most patients (78.5 %) presented with decreased amount of NO2–-NO3–.The number of desquamated endothelial cells was significantly increased in all examined patients compared to control. Comparing endothelial function parameters depending on G894T polymorphism in the eNOS gene, 43.6 % patients with a homozygous genotype for rare T allele were found to have a significant reduction of NO2–-NO3– content compared to control group (p < 0.05). Conclusions. There were significantly more pronounced disorders of the endothelial function in patients with moderate to severe DDSP on the background of type 2 DM. The results of the investigation showed the probable effect of G894T polymorphism of eNOS gene on the formation of a phenotype predisposing to the development of microangiopathies.
цукровий діабет 2го типу; дистальна симетрична полінейропатія; ендотеліальна дисфункція; поліморфізм гена G894T
сахарный диабет 2го типа; дистальная симметричная полинейропатия; эндотелиальная дисфункция; полиморфизм гена G894T
type 2 diabetes mellitus; distal symmetric polyneuropathy; endothelial dysfunction; G894T polymorphism
Introduction
The World Health Organization recognizes diabetes mellitus (DM) as a noninfectious epidemic. It is known that the pathogenetic basis for the formation of vascular complications of type 2 DM is the development of endothelial dysfunction. In Ukraine, more than 1.3 million patients are officially registered, and the amount of patients with type 2 DM is about 90–95 % of all cases of diabetes mellitus (official data of the Ministry of Health of Ukraine, 2013). The significance of this amount keeps impressing. According to the results of certain epidemiological studies have been conducted in our country recently, for each identified patient with type 2 DM there are 2–3 undetected, which limits the possibility of early diagnosis of the disease and prevention of its complications [1].
One of the most common and difficult things in the treatment of diabetic complications is diabetic distal symmetric polyneuropathy (DDSP). According to various authors’ data, DDSP prevalence varies from 15.5 to 77.6 % in the absence of other causes of its occurrence [2, 3].
It is known that the clinical–diagnostic state of DDSP depends on many factors, and the intensity of manifestation of pathological changes is determined genetically [4].
The understanding of the role of the endothelium in the regulation of vascular tone and the mechanism of disorders in this pathology suggests that the endothelium becomes a direct target for damage. The leading role in the formation of endothelial dysfunction belongs to the damaging effects of hyperglycemia, which are connected with non–enzymatic glycosylation of proteins, the polyol pathway of glucose metabolism, and the ability of hyperglycemia to induce free radical oxidation [5–7].
Endothelial nitric oxide synthase (eNOS) in endothelial cells increases the production of NO and cyclic guanosine-–3’,5’–monophosphate in the smooth muscle cells, reducing the effect of vascular congestion caused by phenylephrine and providing endothelial–dependent relaxation in response to acetylcholine [8–11]. Taking into account the physiological role of eNOS and data from previous studies, we hypothesized that the study of the frequency distribution of the genotypes of eNOS–G894T polymorphism in patients with DDPS combined with type 2 DM will allow clarify the pathogenetic mechanisms of the disease, and in the future, molecular genetic data may be useful for justi–fying the tactics of the management of patients with this pathology.
The purpose of the study is to examine the indicators of endothelial function in patients with diabetic distal symmetric polyneuropathy on the background of type 2 diabetes mellitus, which depends on the polymorphism of the endothelial NO synthase gene.
Materials and methods
One–hundred and ten patients with DDSP on the background of type 2 DM were examined and composed the basic group. The average age of patients was 54.2 years (from 38 to 72 years). There were 62 men and 48 women. The comparison group included 80 apparently healthy humans aged from 32 to 56, including 36 men and 44 women.
The patients were distributed according to DDSP severity: the first group consisted of 32 (29.1 %) patients with the initial manifestations of DDSP; the second group included 58 (52.7 %) patients with moderate degree; the third group consisted of 20 (18.2 %) patients with severe DDSP. All patients underwent neurological examination using the scale of neuropathic symptomatic and neuropathic functional counting [12–14].
The endothelial function was investigated by the content of stable metabolites of NO in the blood and the number of circulating endothelial cells in the blood. In this case, the amount of blood circulating endothelial cells was determined by J. Hladovec method modified by N.N. Petrishchev et al. The content of stable metabolities of NO in the blood (nitrites, nitrates) was examined by the content of nitrite–anion (NO2–) and the amount of NO2–, and nitrate–anion (NO3–) in the serum of venous blood using photocolorimetric method of L.C. Green et al.
DNA was isolated from blood cells using the DNA–sorb–B (AmpliSens, Russia). ENOS–G894T polymorphism was detected by polymerase chain reaction (PCR). Allelic polymorphism was determined by gene amplification, followed by Есо24І. Fragments of amplified DNA were separated using gel electrophoresis. DNA was visualized using an ultraviolet emitter. The selection of primers for amplification and subsequent sequencing, as well as PCR analysis, was carried out according to generally accepted world methods.
Statistical analysis was carried out using applications MS Excel 2003, Biostat, Statistica 6. Probability of the received data was calculated by the method of paired test using Student’s t–test. The probable difference in the distribution of samples was determined according to the χ2 criterion. Value of р < 0.05 was considered probable.
Results
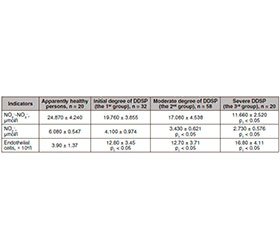
According to the data, most patients (78.2 %) de–monstrated a decrease in the amount of NO2––NO3–, which was detected in all patients with severe DDSP, as well as in 47 (81 %) patients with moderate degree of DDSP and in 17 (53 %) patients with initial manifestations of DDSP that indicated probable decrease in the content of endothelial vasoactive substances with diffusion properties along with the progression of damage to peripheral nerves.
Table 1 demonstrates the comparative evaluation of stable metabolites of nitric oxide in patients with type 2 DM depending on DDSP severity.
It was found that in the examined patients with initial to moderate degree of DDSP, NO2––NO3– level did not differ from that of healthy individuals; in those groups of patients, an increase in the relevant rate was observed in 8 (25 %) patients of the 1st group and in 11 patients (19 %) of the 2nd group.
That was due to the activation of the inductive NO–synthase, which generates toxic to the cell following an excess of NO with the rapid formation of peroxynitrite [11]. The patients with severe DDSP are likely to have moderate decrease of NO indicators by 2.1 times (p < 0.05) compared to the indicators in apparently healthy individuals.
A decrease of this parameter was observed in patients with moderate DDSP by 1.8 times (p < 0.05) and with severe DDSP — by 2.2 times (p < 0.05) in a comparative analysis of NO2 level compared to control group.
It is known that the endothelium performs a barrier function, participates in the regulation of vascular tone, hemostasis, immune response and angiogenesis [15–17]. Restoration of the vascular wall, dysfunction, and desquamation of the endothelium occur due to various pathological processes. The determination of the number of desquamated (circulating) endothelial cells in the blood can be used as one of the indicators of damage to the endothelium.
According to the results of our investigation, the number of desquamated endothelial cells was significantly increased in all examined patients compared with IHI, which also had a slight desquamation of endothelial cells, that might reflect the process of cleansing of intima from dead cells [13]. In patients with initial manifestations of DDSP, the number of endothelial cells was 3.3 times (p < 0.05) higher, with a moderate degree — 3.2 times higher (p < 0.05) and with severe DDSP — 4.3 times higher (p < 0.01) compared to the control group.
The role of ED in the development and progression of diabetic neuropathy was indicated by the established inverse correlation between the concentration of NO2––NO3–, NO2– according to NDS (r = –0.372; р < 0.01 and r = –0.206; р < 0.01) and NSS scales (r = –0.342; р < 0.01 and r = –0.218; р < 0.01).
Discussion
When analyzing the polymorphism, the length of the restriction fragments allowed detect three genotypes of eNOS–G894T polymorphism in analysed genomic DNA samples. It was established that the genetically determined risk for development of the pronounced manifestation of DDSP in patients with type 2 DM (p ≤ 0.05) was associated with the presence of a homozygous genotype with a rare allele T in eNOS gene, as most patients with this genotype had severe DDSP (χ2 = 4.568; p = 0.033). In addition, the frequency of the minor T allele in the basic group of the examined patients was higher (47.2 %) compared to the comparison group (36.9 %).
The Table 2 presents frequency distribution of the genotypes of eNOS–G894T polymorphism, depending on DDSP severity: in patients with initial DDSP manifestations, the homozygous GG genotype of the eNOS gene was found in 31.3 %, heterozygous GT — in 53.1 %, and the homozygous genotype with a rare TT allele was registered in 15.6 %. In patients with moderate severity, genotypes were distributed as follows: GG — in 37.9 %, GT — in 46.5 %, and TT — in 15.5 %; in severe DDSP, the homozygous GG genotype was observed in 50 % of patients, the heterozygous genotype GT — in 15 %, and the homozygous TT genotype for the rare allele was found in 35 % of patients.
Table 3 presents a comparative estimation of NO indicators in patients depending on the distribution of genotypes. The amount of NO2––NO3– varied as follows: in patients with GT and GG genotypes, it did not differ compared to the apparently healthy individuals. A significant decrease in the content of NO2–– NO3– by 43.6 % (р < 0.05) was observed in patients with a homozygous genotype for the rare allele as compared to the control group, indicating a decrease in the content of endothelial vasoactive substances with diffusion properties.
The indicators of NO2– in the examined patients, depending on the distribution of genotypes in patients with GT and GG genotypes, did not differ from the control group, and in patients with TT genotype, its lowest values were observed, which were decreased by 37.5 % (p < 0.05) compared to IHI.
Comparing the number of desquamated endothelial cells in the basic group of patients, depending on the distribution of genotypes (Table 3), it was found that their level was significantly increased in all subjects compared to IHI: in patients with GT genotype — by 69.0 % (p < 0.01), with GG genotype — by 64.5 % (p < 0.05), and in patients with TT genotype the number of desquamated endothelial cells was the highest and increased by 76.8 % (p < 0.01).
However, comparative assessment of this indicator in groups of patients depending on the distribution of genotypes did not detect probable differences.
Conclusions
1. Patients with moderate to severe distal symmetric polyneuropathy on the background of type 2 diabetes mellitus have more severe endothelial dysfunction by reducing basal secretion of NO and a significant increase in the number of desquamated endothelial cells in the blood compared to patients with initial degree of polyneuropathy.
2. The obtained data indicate the potential effect of eNOS–G894T polymorphism on the formation of a phenotype predisposing to the development of microangio–pathies, with the replacement of guanine and thymine, and as a result of glutamate replacement by aspartate in the 298th position of the protein of endothelial NO synthase. This leads to a decrease in NO and the development of endothelial dysfunction, which is an important pathogenetic section in the development of diabetic polyneuropathy in patients with type 2 diabetes mellitus.
Conflicts of interests. Authors declare no conflicts of interests that might be construed to influence the results or interpretation of their manuscript.

/89-1.jpg)
/90-1.jpg)