Журнал «Здоровье ребенка» Том 14, №3, 2019
Вернуться к номеру
Показники нітрозативного та оксидантного стресу як неінвазивні маркери бактеріального менінгіту в недоношених дітей
Авторы: G.O. Lezhenko(1), O.Ye. Pashkova(1), A.V. Abramov(1), L.O. Volotko(2)
(1) — Zaporizhzhia State Medical University, Zaporizhzhia, Ukraine
(2) — MI “Zaporizhzhia Regional Clinical Children’s Hospital” of ZRС, Zaporizhzhia, Ukraine
Рубрики: Педиатрия/Неонатология
Разделы: Клинические исследования
Версия для печати
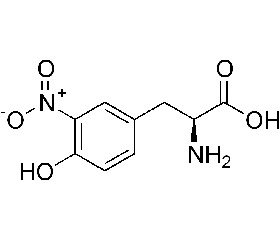
Актуальність. В умовах оксидантного та нітрозативного стресу головний мозок уразливий для окисного пошкодження через високе споживання кисню, велику кількість заліза, відносно низький рівень експресії антиоксидантів, високий вміст поліненасичених жирних кислот. Один із маркерів нітрозативного стресу — нітротирозин. Високі концентрації нітротирозину в лікворі пов’язані з несприятливим перебігом захворювання. Як індикатор інтенсивності оксидантного стресу використовується також гомоцистеїн. Підвищені концентрації гомоцистеїну мають цитотоксичну дію, що призводить до загибелі клітини. Мета дослідження: вивчення показників нітрозативного та оксидантного стресу при бактеріальних менінгітах у недоношених дітей. Матеріали та методи. Обстежено 52 новонароджених. Основна група — 14 недоношених дітей (середній термін гестації 31,7 ± 0,9 тижня), хворих на бактеріальний менінгіт. Група порівняння — 20 новонароджених із перинатальними ураженнями ЦНС (10 недоношених із середнім терміном гестації 32,3 ± 0,4 тижня та 10 доношених новонароджених). Контрольна група — 18 здорових доношених дітей. Проводили оцінку анамнезу, соматичного та неврологічного статусу новонароджених, даних нейросонографіі. Методом імуноферментного аналізу досліджували вміст нітротирозину в сироватці крові. Результати. Встановлено, що вміст нітротирозину в сироватці крові новонароджених основної групи був вірогідно нижчим, ніж у групі порівняння та контрольній групі (1,38 ± 0,06 проти 1,53 ± 0,07 ммоль/л та 1,61 ± 0,04 ммоль/л відповідно; р < 0,05), залежав від гестаційного віку дитини (r = +0,63; p < 0,05). Уміст гомоцистеїну в сироватці обернено залежний від гестаційному віку дитини (r = –0,62; p < 0,05) та в новонароджених основної групи статистично перевищував показники в групі порівняння та контрольній групі (17,10 ± 1,13 проти 10,80 ± 1,10 мкмоль/л та 9,56 ± 1,42 мкмоль/л відповідно, p < 0,05). Висновки. Низькій вміст у сироватці крові нітротирозину та гіпергомоцистеїнемія можуть сприяти ураженню судинного русла, проникненню мікроорганізмів через гематоенцефалічний бар’єр та розвитку інфекційного процесу. Максимальний вміст гомоцистеїну в сироватці крові спостерігається при розвитку бактеріального менінгіту.
Актуальность. В условиях оксидантного и нитрозативного стресса мозг уязвим для окислительного повреждения вследствие высокого потребления кислорода, большого количества железа, относительно низкого уровня экспрессии антиоксидантов, высокого содержания полиненасыщенных жирных кислот. Один из маркеров нитрозативного стресса — нитротирозин. Высокие концентрации нитротирозина в ликворе связаны с неблагоприятным течением заболевания. В качестве индикатора интенсивности оксидантного стресса используется также гомоцистеин. Повышенные концентрации гомоцистеина имеют цитотоксическое действие, что приводит к гибели клетки. Цель исследования: изучение показателей нитрозативного и оксидантного стресса при бактериальных менингитах у недоношенных детей. Материалы и методы. Обследовано 52 новорожденных. Основная группа — 14 недоношенных детей (средний срок гестации 31,7 ± 0,9 недели), больных бактериальным менингитом. Группа сравнения — 20 новорожденных с перинатальными поражениями ЦНС (10 недоношенных детей со средним сроком гестации 32,3 ± 0,4 недели и 10 доношенных новорожденных). Контрольная группа — 18 здоровых доношенных детей. Проводили оценку анамнеза, соматического и неврологического статуса новорожденных, данных нейросонографии. Методом иммуноферментного анализа было исследовано содержание нитротирозина в сыворотке крови. Результаты. Установлено, что содержание нитротирозина в сыворотке крови новорожденных основной группы было достоверно ниже, чем в группе сравнения и контрольной группе (1,38 ± 0,06 против 1,53 ± 0,07 ммоль/л и 1,61 ± 0,04 ммоль/л соответственно; р < 0,05), зависело от гестационного возраста ребенка (r = +0,63; p < 0,05). Содержание гомоцистеина в сыворотке обратно пропорционально гестационному возрасту ребенка (r = –0,62; p < 0,05) и у новорожденных основной группы статистически превышало таковое в группе сравнения и контрольной группе (17,10 ± 1,13 против 10,80 ± 1,10 и 9,56 ± 1,42 мкмоль/л соответственно; p < 0,05). Выводы. Низкое содержание в сыворотке крови нитротирозина и гипергомоцистеинемия могут способствовать поражению сосудистого русла, проникновению микроорганизмов через гематоэнцефалический барьер и развитию инфекционного процесса. Максимальное содержание гомоцистеина в сыворотке крови наблюдается при развитии бактериального менингита.
Background. In oxidative and nitrosative stresses, the brain is vulnerable to oxidant damage due to high oxygen consumption, large amounts of iron, relatively low antioxidant expression, high concentration of polyunsaturated fatty acids. One of the markers of nitrosative stress is nitrotyrosine. Cerebrospinal fluid high concentration of nitrotyrosine is associated with the adverse course of the disease. Homocysteine is also considered as an indicator of the intensity of oxidative stress. Increased concentrations of homocysteine have cytotoxic effects that leads to cell death. Materials and methods. The study included 52 preterm infants. The basic group consisted of 14 preterm infants (mean gestation period 31.7 ± 0.9 weeks) with bacterial meningitis. The experimental group included 20 newborns with the perinatal disorders of the central nervous system (10 preterm infants with an average gestational age of 32.3 ± 0.4 weeks and 10 full-term newborns). Control group consisted of full-term healthy 18 newborns. Anamnesis, somatic and neurological status of newborns, neurosonography data were evaluated. The nitrotyrosine blood serum concentration was evaluated using enzyme-linked immunosorbent assay. Results. The results of the study demonstrated that nitrotyrosine content in the blood serum of newborns in the basic group was significantly lower than in the experimental and control groups (1.38 ± 0.06 vs 1.53 ± 0.07 and 1.61 ± 0.04 mmol/L, respectively, p < 0.05) and depended on the gestational age of a child (r = +0.63; p < 0.05). The homocysteine level in blood serum had an inverse correlation with a gestational age of the child (r = –0.62; p < 0.05) and in newborns in the main group statistically exceeded the rates of newborns in the experimental group and the control group (17.10 ± 1.13 versus 10.80 ± 1.10 and 9.56 ± 1.42 μmol/L, respectively; p < 0.05). Conclusions. Low levels of nitrotyrosine in serum and hyperhomocysteinemia observed in preterm infants may contribute to damage to the vascular bed, penetration of microorganisms through the blood-brain barrier and the development of the infectious process. The maximum content of homocysteine in blood serum is observed with the development of bacterial meningitis.
новонароджені; менінгіт; нітротирозин; гомоцистеїн
новорожденные; менингит; нитротирозин; гомоцистеин
newborns; meningitis; nitrotyrosine; homocysteine
Introduction
Nowadays a high level of neonatal death from an infection remains an urgent challenge, and therefore, diagnosis of infectious-inflammatory diseases in infants is of great importance [1]. Among all infectious diseases in newborns, meningitis ranks high; morbidity from it varies from 0.26 to 0.46–0.50 per 1000 newborns according to the data of different authors. Thus, up to 80 % of all cases of meningitis are diagnosed in preterm infants [2, 3]. The leading role of oxidative and nitrosative stress is presently well-proven in the physiopathology of bacterial meningitis [4, 5]. Under conditions of oxidative and nitrosative stress, the brain becomes particularly vulnerable to oxidative damage due to its high oxygen consumption, high amount of iron, low level of antioxidant expression, and high content of polyunsaturated fatty acids [6, 7]. Nitrotyrosine is one of the markers of nitrosative stress [8]. The reports demonstrated the patients with meningitis to have tyrosine nitration significantly increased, thus the high concentrations of nitrotyrosine in cerebrospinal fluid were related to the unfavorable course of disease [9]. Homocysteine and its derivatives can be also used as the indicators of the intensity of oxidative stress [10]. Increased homocysteine concentrations associated with oxidative stress development have a cytotoxic effect that causes the death of cells [11]. The experiment showed that hyperhomocysteinemia stimulates inflammatory response induced by the expression of pro-inflammatory cytokines and assists the accumulation of monocytes and macrophages in the wall of vessels [12, 13]. In spite of considerable successes with diagnosis and treatment of meningitis in newborns, research of indices of nitrosative and oxidative stress in bacterial meningitis in preterm infants is still relevant, so as it can result in the development of advanced approaches to their treatment.
The purpose was to study of indicators of nitrosative and oxidative stress in bacterial meningitis in preterm infants.
Materials and methods
The study included 52 newborns. The basic group included 14 preterm infants with bacterial meningitis (the average gestation period was 31.7 ± 0.9 weeks). The diagnosis of meningitis was set based on the clinical and laboratory criteria. Experimental group included 20 newborns with the perinatal disorders of CNS, among which there were 10 preterm infants (the average gestation period 32.3 ± 0.4 weeks) and 10 newborn infants (the average gestational age 38.7 ± 0.5 weeks). The control group included 18 newborn infants with an average gestational age 38.6 ± 0.3 weeks without disorders of the CNS and inflammatory diseases.
All infants were evaluated for the ante- and intranatal anamnesis, the somatic status of newborns, the main syndromes of perinatal injury of the brain, data from neurosonography. Neurosonography was performed in the first two days of staying in a hospital on the device “Medison SA 8000 Live” with a convective linear sensor of 5 MHz. Additionally, the content of nitrotyrosine in blood serum was determined on an immunoenzyme assay (IEA) using commercial kits “Nitrotyrosine”, ELISA (Hycult Biotech) and homocysteine, Axis® Homocysteine EIA (UK).
Statistical data analysis was performed using the software package Statistica 13.0 (StatSoftInc., No JPZ8041382130ARCN10-J) with the calculation of the arithmetic mean (M), standard deviation (s) and mean errors (m). The correlation between individual factors was estimated using Pearson correlation coefficient. To assess differences of the indicators in the compared groups, Student’s t-test was used. Differences were considered significant at p < 0.05.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Declaration of Helsinki, 1964, and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study. The full data set by infants’ parents and physician that support the findings of this study are not publicly available due to the restrictions of the ethics approval originally obtained.
Results
Analysis of the data showed that in the basic and experimental groups, most children were born with asphyxia (92.8 and 85.0 %, respectively, p > 0.05), which is significantly more frequent than in the control group (4 children, 22.2 %; p < 0,05). However, among preterm infants, who had been diagnosed with bacterial meningitis, asphyxia of moderate to severe degree (64.3 %) with an Apgar score of 2–5 was more often recorded, whereas in the experimental group only 3 (15.0 %) of 20 children were born with an Apgar score of 5, while other children were rated at birth 7–8 points.
Clinically, according to the results of the assessment of neurological status, in the group of newborns with bacterial meningitis, the central nervous system depression syndrome predominated (13 (92.8 %) of 14 newborns) which is 3.7 times more often than in the experimental group (25.0 %; p < 0.05). In the clinical picture, these children showed a decrease in neuro-reflex activity, muscle hypotonia, and decreased spontaneous motor activity. Unlike the patients in the basic group, the syndrome of hyperexcitability (75.0 %) was reported more often in the newborns of the experimental group; it was characterized by increased motor activity, tremor of the extremities and chin, increased oral and spinal unconditioned automatisms, and emotional anxiety.
Hypertensive-hydrocephalic syndrome significantly prevailed in bacterial meningitis preterm infants (71.4 %) compared with the newborns with perinatal CNS disorders (25.0 %; p < 0.05).
The convulsive syndrome in the preterm group occurred in 9 (64.3 %) infants and only in 3 (15.0 %) children in the experimental group. Convulsions were mainly focal or multifocal and accompanied by respiratory rate disorders, the apnea development, a decrease in muscle tone and neuro-reflex activity.
The neurosonography results showed that all newborns of the basic group evidenced an increase in the echogenicity of the parenchyma of the brain. Six (42.8 %) patients with meningitis also presented with increased echogenicity and expansion of brain furrows, in concert with the accumulation of liquid in the interhemispheric fissure. The results of the study demonstrated thickening and increased echogenicity of lateral ventricles with additional inclusions determined in the ventricular cavity, fuzziness or deformation of contours of vascular plexuses and the expansion of the ventricular system which testified to the development of inflammatory process of the ventricular system on the background of meningitis, that is ventriculitis, in 8 (57.4 %) newborns of the basic group.
Neurosonography data in the experimental group were characterized by homogeneous echogenicity increase of the periventricular region. These changes were due to the immaturity of the brain structure and decreased while observing. Eight (40.0%) newborns of this group experienced an increase in the echogenicity of the parenchyma of the brain and basal ganglia, the fuzzy visualization of the furrows and brain flexures, and the absence of pulsations in the vessels. Against this backdrop, narrowing and fuzzy visualization of the lateral ventricles were observed due to edema. Peri- and intraventricular hemorrhages of varying degrees of severity were revealed in 11 (91.0 %) newborns of the basic group and in 5 (25.0 %) newborns in the experimental group associated in all cases with muscular hypotension, the disappearance of sucking and swallowing reflexes, decreased motor activity. Five (35.7 %) newborns with intraventricular hemorrhage in the basic group presented with attacks of apnea and arrhythmias.
Changes in cerebrospinal liquor in newborns in the basic group were characterized by elevated cytosis (144.5 ± 42.9 cells/μl) of neutrophilic nature (79.4 ± 6.5 %), the protein level was 0.72 ± 0.09 g/l. Deviations in the content of the liquor in the experimental group were not detected. In 9 (64.3 %) newborns with meningitis and in 5 (25.0 %) newborns in the experimental group the complete blood count found decreased number of red blood cells, hemoglobin and hematocrit. Taking into account the pathological injury of the CNS, inflammatory processes of any genesis arise in the conditions of the development of nitrosative and oxidative stress [14], the next stage of our work was to study the content of nitrotyrosine and homocysteine in the infants’ blood serum in studied groups.
According to the results of the research, it was found that nitrotyrosine content in the blood serum of newborns in the basic group was significantly lower than its level in the control and experimental groups (Table 1).
Given that the findings contradict the traditional concept that meningitis provokes oxidative and nitrosative stress caused by active forms of nitrogen and altered antioxidant protection, which may result in increased nitrotyrosine [9], we have assumed that the content of the latter depends on the gestational age of an infant. Subsequently, the results of the pair correlation analysis (r = +0.63; p < 0.05) allowed us to confirm our assumption. That is in preterm infants, the lowest levels of nitrotyrosine were observed. Therefore, at the next stage, we analyzed the nitrotyrosine content in the experimental group depending on the gestational age of a newborn.
The findings of the analysis of the obtained levels of nitrotyrosine in the blood serum of newborns of the experimental group, depending on the period of gestation demonstrated the following. We observed its low values in preterm infants compared to the indices of full-term infants of this group and control group (Table 2).
Against this backdrop, it was noted that although the development of meningitis in preterm infants was associated with increased production of nitrotyrosine, its rates did not reach the values of full-term infants. That is, in preterm infants, even in the presence of the inflammatory process, there was no increase in blood serum nitrotyrosine even to the level of full-term infants.
The content of homocysteine in infants in the basic group was statistically significantly higher than that of the newborns in the experimental and control groups. Like nitrotyrosine, the level of homocysteine also depended on the gestational age of a child, but had a reverse correlation (r = –0.62, p < 0.05), that is, its highest concentration was observed in children with less gestational age. However, the maximum values of homocysteine were registered in the group of preterm infants with meningitis.
Discussion
Nitrotyrosine is known to be the ultimate stable product of nitric oxide, which is formed by nitrating tyrosine with peroxynitrite [15, 16]. The increase in the production of nitric oxide in newborns, on the one hand, serves as a compensatory and adaptive reaction aimed at preserving the processes of microcirculation of hemodynamics in organs and systems [17], and on the other hand, high NO concentrations exhibit antimicrobial properties due to its cytotoxic action, which is based on the reaction of nitrosylation with the formation of peroxynitrite [18]. Taking into account that vascular injuries in neuroinfectious diseases are an obligatory pathogenetic link due to the predominant haematological distribution of pathogens [19], it can be assumed that in preterm infants there is an insufficient synthesis of nitric oxide and, consequently, a decrease in the synthesis of nitrotyrosine, which may be one of the reasons for the development of microcirculation in the brain. Taking into account that nitrotyrosine, like nitric oxide, affects the immune response by its activation at the expense of products of immunoglobulins that recognize it [20, 21], the inadequate synthesis of the latter can contribute to the penetration of pathogenic microorganisms through the blood-brain barrier, as well as protracted the course of the infectious process. Hyperhomocysteinemia, which leads both to a decrease in the synthesis of NO and to direct NO degradation, is another factor that may contribute to the disturbance of normal production of endothelial cells from NO to endothelial cells and to reduce its bioavailability in preterm infants [22, 23]. Perhaps, a certain explanation of the facts obtained is the results of the experimental work of Da Cunha et al. (2012) in rats where it was shown that hyperhomocysteinemia increases the content of nitrites in the hippocampus with their simultaneous reduction in blood serum [24]. The consequence of high concentrations of homocysteine is damage to cerebral vessels, which leads to the development of vasculopathies or vasculitis, induction of apoptosis of neurons, accompanied by the development of inflammatory reaction and violation of the blood-brain barrier [25, 26]. At the same time, hyperhomocysteinemia leads to an increase in the level of mediators of inflammation, both in the blood and in tissues, including the brain [24, 27].
Conclusions
Thus, the obtained results indicate that low nitrotyrosine levels and hyperhomocysteinemia occur in preterm infants, which may contribute to vascular tract damage, penetration of microorganisms through the blood-brain barrier and the development of the infectious process. The maximum content of homocysteine in blood serum is observed with the development of bacterial meningitis. At the same time, in preterm infants with bacterial meningitis, inadequate activation of the synthesis of nitrotyrosine is established which may be one of the causes of a prolonged course of the disease. However, at the present stage, questions relating to the pathogenetic mechanisms of bacterial meningitis in preterm infants are not fully developed and need further development.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
1. Цораева З.А. Показатели иммунного статуса у новорожденных недоношенных детей с инфекционно-воспалительными заболеваниями / З.А. Цораева, Т.Б. Касохов, А.Н. Шляйхер // Современные проблемы науки и образования. — 2016. — № 2. — С. 133.
2. Михалев Е.В. Клинико-патогенетические аспекты гнойного менингита у недоношенных новорожденных с гипоксическим поражением центральной нервной системы : автореф. дис. на соискание науч. степени д-ра мед. наук : спец. 14.00.09 «Педиатрия» / Михалев Е.В.; ГОУВПО «Сибирский государственный медицинский университет». — Томск, 2005. — 46 с.
3. Прилуцкая В.А. Бактериальные менингиты у новорожденных детей / В.А. Прилуцкая. — Минск : Белорусский государственный медицинский университет, 2011. — 48 с.
4. Barichello T. Oxidative stress, cytokine/chemokine and disruption of blood-brain barrier in neonate rats after meningitis by Streptococcus agalactiae / T. Barichello, J.C. Lemos, J.S. Generoso // Neurochem. Res. — 2011. — № 36. — Р. 1922-1930. doi: 10.1007/s11064-011-0514-2.
5. Giridharan V.V. Temporal changes of oxidative stress markers in Escherichia coli K1-induced experimental meningitis in a neonatal rat model / V.V. Giridharan, L.R. Simões, V.S. Dagostin, J.S. Generoso, G.T. Rezin, D. Florentino, J.P. Muniz et al. // Neurosci. Lett. — 2017. — № 653. — P. 288-295. doi: 10.1016/j.neulet.2017.06.002.
6. Harris R.A. Sweet and sour-oxidative and carbonyl stress in neurological disorders / R.A. Harris, S. Amor // CNS Neurol. Disord. Drug Targets. — 2011. — № 10 (1). — Р. 82-107.
7. Klein M. Oxidative stress in pneumococcal meningitis: a future target for adjunctive therapy? / M. Klein, U. Koedel, H.W. Pfister // Prog. Neurobiol. — 2006. — № 80 (6). — Р. 269-280.
8. Darwish R.S. Nitrotyrosine as an oxidative stress marker: evidence for involvement in neurologic outcome in human traumatic brain injury / R. Darwish, N. Amiridze, B. Aarabi // J. Trauma. — 2007. — Vol. 63, № 2. — Р. 439-442.
9. Kastenbauer S. Oxidative stress in bacterial meningitis in humans / S. Kastenbauer, U. Koedel, B.F. Becker, H.W. Pfister // Neurology. — 2002. — № 58. — Р. 186-191.
10. Окислительный стресс. Прооксиданты и антиоксиданты / Е.Б. Меньщикова, В.З. Ланкин, Н.К. Зенков, И.А. Бондарь, Н.Ф. Круговых, В.А. Труфакин. — М.: Слово, 2006. — 556 с.
11. Sipkens J.A. Homocysteine-Induced Apoptosis in Endothelial Cells Coincides With Nuclear NOX2 and Peri-nuclear NOX4 Activity / J.A. Sipkens, N. Hahn, C.S. van den Brand, C. Meischl, S.A. Cillessen, D.E. Smith, L.J. Juffermans et al. // Cell Biochem. Biophys. — 2013. — № 67. — Р. 341-352. doi: 10.1007/s12013-011-9297-y.
12. Meng S. Homocysteine induces inflammatory transcriptional signaling in monocytes / S. Meng, S. Ciment, M. Jan, T. Tran, H. Pham, R. Cueto, X.F. Yang et al. // Front. Biosci. — 2013. — № 18. — Р. 685-95.
13. Zeng X. Homocysteine mediated expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human monocytes / X. Zeng, J. Dai, D.G. Remick, X. Wang // Circ. Res. — 2003. — № 93. — Р. 311-320.
14. Беленичев И.Ф. Нейропротекция и нейропластичность / И.Ф. Беленичев, В.И. Черний, Е.А. Нагорная. — К.: ООО «Полиграф плюс», 2014. — 512 с.
15. Анаев Э.Х. Биологические маркеры при хронической обструктивной болезни легких / Анаев Э.Х. // Практическая пульмонология. — 2018. — № 1. — С. 26-32.
16. Shiffrin E.L. Oxidative stress, nitric oxide synthase, and superoxide dismutase / E.L. Shiffrin // Hypertension. — 2008. — № 51. — Р. 31-32. doi: 10.1161/hypertensionaha.107.103226.
17. Гафарова Ф.М. Система оксида азота в крови новорожденных с повышенным перинатальным риском / Ф.М. Гафарова // Вестник Российской академии медицинских наук. — 2010. — № 3. — С. 36-40.
18. Beckmann J.S. Nitric oxide, superoxide and peroxynitrite: the good, the bad and ugly / J.S. Beckmann, W.H. Koppenol // Am. J. Physiol. — 1996. — № 271. — Р. 1424-1437. doi: 10.1152/ajpcell.1996.271.5.C1424.
19. Скрипченко Н.В. Клинико-лучевая диагностика церебральных васкулитов при нейроинфекциях у детей / Н.В. Скрипченко, Т.Н. Трофимова, Е.С. Егорова, Н.В. Моргацкий, Е.А. Космачева // Российский вестник перинатологии и педиатрии. — 2010. — № 1. — С. 101-106.
20. Oгородова Л.М. Влияние 3-нитротирозина на формирование субпопуляции Т-регуляторных клеток при воспалении дыхательных путей / Л.М. Огородова, И.В. Петрова, К.Ю. Рукин // Научные ведомости Белгородского государственного университета. — 2011. — № 15 (6). — С. 78-82.
21. Ischiropoulos H. Protein tyrosine nitration an update / H. Ischiropoulos // Arch. biochem. biophys. — 2009. — № 484. — С. 117-121. doi: 10.1016/j.abb.2008.10.034.
22. Нестеренко О.В. Гипергомоцистеинемия у детей с пиелонефритом / О.В. Нестеренко, С.Р. Утц, В.Б. Бородулин, В.И. Горемыкин, С.Ю. Елизарова, Е.В. Бобылева, Ю.М. Моисеева и др. // Российский вестник перинатологии и педиатрии. — 2016. — № 4. — С. 88-92.
23. Kraus J.P. Biochemistry and molecular genetics of cystathionine beta-synthase deficiency / J.P. Kraus // Eur. J. Pediatr. — 1998. — № 157. — Р. 50-53.
24. Da Cunha A.A. Chronic hyperhomocysteinemia increases inflammatory markers in hippocampus and serum of rats / A.A. da Cunha, A.G. Ferreira, S.O. Loureiro, M.J. da Cunha, F. Schmitz, C.A. Netto, A.T. Wyse // Neurochem. Res. — 2012. — № 37. — Р. 1660-1669. doi: 10.1007/s11064-012-0769-2.
25. Березовская Т.С. Диагностическое значение определения гомоцистеина в сыворотке крови у детей при нейроинфекциях / Т.С. Березовская, Н.А. Мироманова // Журнал инфектологии. — 2018. — Т. 10, № 1. — С. 42-46.
26. Zhang Y. The immunomodulatory mechanism of brain injury induced by hyperhomocysteinemia in spontaneously hypertensive rats / Y. Zhang, L. Wang, X. Zhou, J. Geng, X. Li // J. Cell. Biochem. — 2019. — № 120. — Р. 9421-9429. doi: 10.1002/jcb.28217.
27. Da Cunha A.A. Increased inflammatory markers in brain and blood of rats subjected to acute homocysteine administration / A.A. da Cunha, A.G. Ferreira, A.T. Wyse // Metab. Brain Dis. — 2010. — № 25. — P. 199-206. doi: 10.1007/s11011-010-9188-8.

/16-1.jpg)