Журнал "Гастроэнтерология" Том 53, №2, 2019
Вернуться к номеру
Визначення міжклітинних контактів у хворих із синдромом подразненого кишечника
Авторы: Yu.M. Stepanov(1), I.Ya. Budzak(2), Yu.A. Gaidar(1)
(1) — State Institution “Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine”, Dnipro, Ukraine
(2) — State Institution “Dnipropetrovsk Medical Academy of the Ministry of Health of Ukraine”, Dnipro, Ukraine
Рубрики: Гастроэнтерология
Разделы: Клинические исследования
Версия для печати
У статті наведено аналіз літературних даних стосовно порушення кишкової проникності як одного з патогенетичних факторів синдрому подразненого кишечника. Детально розглянуто будову щільних контактів як важливого елемента нормального функціонування кишкового бар’єра. Розглянуті дослідження, у яких попередньо вивчали експресію компонентів щільних контактів у хворих із синдромом подразненого кишечника. Показанo результати власного дослідження з оцінкою експресії оклюдину в пацієнтів iз синдромом подразненого кишечника. Було встановлено, що експресія оклюдину є нижчою у хворих із синдромом подразненого кишечника порівняно зі здоровими особами.
В статье приведен анализ литературных данных о нарушении кишечной проницаемости как одного из патогенетических факторов синдрома раздраженного кишечника. Детально рассмотрено строение плотных контактов как важного элемента нормального функционирования кишечного барьера. Рассмотрены исследования, в которых предварительно изучали экспрессию компонентов плотных контактов у больных с синдромом раздраженного кишечника. Показаны результаты собственного исследования с оценкой экспрессии окклюдина у пациентов с синдромом раздраженного кишечника. Было установлено, что экспрессия окклюдина ниже у больных с синдромом раздраженного кишечника по сравнению со здоровыми лицами.
The article presents an analysis of literature data on the violation of intestinal permeability as one of the pathogenetic factors of irritable bowel syndrome. The structure of tight junctions is considered in detail as an important element of the normal functioning of the intestinal barrier. The researches that previously studied the expression of tight junction components in patients with irritable bowel syndrome were considered. The results of our own study with the evaluation of occludin expression in patients with irritable bowel syndrome are shown. It has been found that the expression of occludin is lower in patients with irritable bowel syndrome compared with healthy individuals.
синдром подразненого кишечника; кишкова проникність; щільні контакти; оклюдин; імуногістохімічний метод
синдром раздраженного кишечника; кишечная проницаемость; плотные контакты; окклюдин; иммуногистохимический метод
irritable bowel syndrome; intestinal permeability; tight junctions; occludin; immunohistochemical method
Introduction
Irritable bowel syndrome (IBS) remains one of the most common diseases of the digestive system today. Despite all the achievements of the last decades, the efficacy of IBS treatment remains inadequate. First of all, this is due to the fact that the modern therapy of IBS has primarily symptomatic direction. It is important to study the causes and pathogenetic factors of the onset and development of IBS with a view to their further correction, which, of course, will significantly improve the effectiveness of the treatment of this disease.
For a long time, the psycho-emotional component was considered perhaps the only cause of IBS. However, studies of the last decades have confirmed that the psycho-emotional factor is sufficiently important and frequent, however, not the only one in the development of IBS. The role of the prior intestinal infection as a factor for developing the so-called post-infectious IBS is proved. More and more studies are available, proving the presence of minimal inflammation in the intestinal mucosa in patients with IBS. The aspect of the relationship between intestinal microflora and IBS is interesting and rapidly developing. The study of other factors for the development of this pathology continues.
The role of intestinal epithelial permeability in patients with IBS is an interesting and promising area in the study of IBS pathogenesis. The intestinal epithelial barrier is an important factor in intestinal homeostasis and is the first line of intestinal protection from external factors (microorganisms, food antigens, etc.). An intercellular integration violation can activate a local immune response that may have an effect on the development of IBS [1–3].
The intestinal epithelial barrier is one of the largest surfaces in the body that contact the external area. And it has a very important task: on the one hand, to ensure unimpeded absorption of water and nutrients, on the other hand, to protect from the harmful effects of bacterial and non-bacterial external factors located in the intestinal cavity. Different protective factors perform this important function in the body; there are two levels of protection: immune (epithelial and immune cells) and non-immune (intestinal motility, mucus layer on the surface of the epithelium, water secretion) ones. All these components of protection normally operate in a complex and consistent manner, and a violation of one of them can disrupt the normal functioning of the intestinal barrier [1].
Among the cells of the intestinal mucosa involved in providing the intestinal epithelial barrier, a number of cells can be noted: enterocytes, mucus-producing goblet cells, enteroendocrine cells, Paneth cells, T- and B-lymphocytes, IgA-secreting plasma cells, mast cells, dendritic cells, macrophages. They closely interact with each other and provide the protective function of the intestinal epithelium. Certain receptors such as toll-like receptors, nucleotide-binding oligomerization domain-like receptors also perform the important protective function of the intestinal epithelium. They protect from harmful factors, co-operate with the beneficial intestinal microflora, and, if necessary, affect the expression of antimicrobial peptides [1].
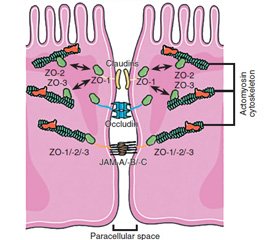
In this aspect, the apical complex on the border between the epithelial cells and the intestinal cavity is of particular interest. It provides a density of contacts between the cells of the intestinal epithelium. The main component of this complex is tight junction. Tight junctions are composed of transmembrane proteins (claudin, occludin, junctional adhesion molecules), which interact with the proteins of adjacent cells. These proteins are connected to the actomyosin cytoskeleton filaments through scaffolding proteins (zonula occludens (ZO)) [1].
Tight junctions are under constant influence of various physiological and pathological factors. Of course, the smooth operation of tight junction components depends to a large extent on the normal functioning of the entire intestinal epithelial barrier.
We have considered in a simplified version the normal functioning of the intestinal epithelial barrier, but the question arises about how this all is related to IBS.
To date, there is ample evidence of the correlation between increased intestinal permeability and IBS, in particular diarrhea-predominant IBS. Back in 2000, R.C. Spiller et al. showed increased intestinal permeability in patients with post-infectious form of IBS caused by a previous infection with Campylobacter enteritis [6].
Similar results were obtained by J.K. Marshall et al. (2004). Lactulose-mannitol test for intestinal permeability was performed in 132 patients with IBS and 86 controls. The result showed a significant increase in patients with IBS compared to controls (Mann-Whitney’s score was 118.8 vs. 95.3, P = 0.007) [7].
In the study of S.P. Dunlop et al. (2006), it was shown that in patients with diarrhea, the permeability of the small cell epithelium was significantly increased compared to patients with constipation-predominant IBS and healthy individuals. Interestingly, in patients with IBS without prior intestinal infection, permeability was more affected than in patients with post-infection type of IBS [4].
Q. Zhou et al. in 2009 investigated the intestinal permeability by means of lactulose-mannitol method in 54 patients with diarrhea, and 22 healthy individuals. In 39 % of patients with diarrhea-predominant IBS, an increased permeability of the intestinal epithelium was detected. The relationship between increased intestinal permeability and the severity of intestinal symptoms was an important result of this study. Thus, the Functional Bowel Disorder Severity Index in patients with IBS and high permeability was 100.8 ± 5.4, in patients with IBS and normal permeability — 51.6 ± 12.7, in controls — 6.1 ± 5.6. The authors of the study concluded that increased intestinal permeability increases visceral hypersensitivity in patients with IBS [5].
Interesting results were recently obtained in the study of Z. Mujagic et al. Small intestinal and gastroduodenal permeability was analyzed by various methods in patients with different forms of IBS as compared to controls. It was found that gastroduodenal permeability was increased in all forms of IBS. Instead, the intestinal permeability was elevated predominantly in patients with diarrhea-predominant IBS [8].
In recent years, there were obtained the results of the evaluation of the tight junction components in patients with IBS.
In a study by P. Cheng et al. (2015), molecular and cellular mechanisms of tight junctions were evaluated using electron microscopy, and intestinal epithelial claudin-1 was detected by immunohistochemical study, western blot analysis, and fluorescence quantitative polymerase chain reaction. Claudin-1 expression has been shown to be reduced in diarrhea-predominant IBS and elevated in IBS with constipation [9]. In another study in China, the following results were shown: claudin-1 and -4 expression decreased in patients with IBS and diarrhea, claudin-1, -3, and -4 expression increased with constipation [10].
In the Spanish study of C. Martínez et al., the expression of ZO-1 in the intestinal biopsy sample was analyzed, taking into account the number of mast cells and clinical symptoms in patients with diarrhea-predominant IBS. It has been found that ZO-1 expression was significantly reduced in patients with diarrhea. There is also a significant correlation between ZO protein, mast cell activity and clinical symptoms in patients with IBS [11].
As already mentioned, with regard to the tight junction components, there is a difference in the various forms of IBS. In a recent study, there was no significant difference in the expression of occludin, claudin, and ZO in women with constipation-predominant IBS compared to controls [12].
In the study by N. Bertiaux-Vandaële et al., all major tight junction components were examined in 55 patients with different forms of IBS. It was found that expression of ZO-1 and occludin was significantly reduced in patients with IBS, while claudin had a tendency to decrease. The study of these indices in different forms of IBS showed that expression of occludin and claudin-1 was reduced in diarrhea-predominant form and was not reduced in constipation-predominant and mixed form of IBS. Expression of occludin and claudin-1 correlated with the duration of IBS symptoms. Interestingly, the expression of occludin was particularly reduced in patients with abdominal pain higher than 6 on the visual analogue scale that confirmed the relationship between the state of tight junction (and, consequently, intestinal permeability) and the severity of clinical symptoms of IBS [13].
We would like to share the results of our own research on the subject.
The purpose of the study was to compare the expression of occludin in patients with IBS and in healthy individuals.
Materials and methods
The research was conducted at the premises of the Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine. Nineteen biopsy specimens were evaluated: 12 — in patients with diarrhea-predominant IBS, 7 — in healthy controls.
The study was performed using immunohistochemical method. As primary antibodies, rabbit polyclonal occludin antibodies (GeneTex, USA-Taiwan) were used at dilution of 1 : 50. Master Polymer Plus Detection System (peroxidase), including DAB Chromogen (Master Diagnostica, Spain), was used as secondary antibodies.
During the immunohistochemical reaction, occludin was stained brown. To determine occludin content, quantitative morphometric analysis was performed using the ImageJ program, which evaluated the brightness of the images in pixels. In every biopsy sample, there were evaluated 10 squares (100 × 100 pixels), as well as minimal, maximal and mean brightness. The darker the image was (the more the occlusion was expressed), the lower the brightness value at ImageJ.
Results
Figures 2 and 3 show biopsy specimens in patients with IBS and in control group.
Parameters of occludin expression in comparison groups are shown in Tables 1 and 2.
As can be seen from the data obtained, the brightness during the ImageJ evaluation in the IBS group (168.30 ± 0.63) was significantly higher than in the control group (158.70 ± 1.46) (p < 0.001). Consequently, occludin expression in patients with IBS was significantly lower than in healthy subjects.
Conclusions
1. The use of an immunohistochemistry to evaluate the expression of occludin in the intestinal mucosa allows us to evaluate the permeability of the intestinal epithelium.
2. In patients with IBS, the expression of occludin is significantly lower than that of healthy individuals.
Thus, one of the most important factors in the development of IBS is the deterioration in the functioning of the intestinal epithelial barrier. The studies performed show a decrease in occludin concentrations in patients with IBS. Further investigations concerning this problem may be very important for better understanding of pathogenesis of IBS.
1. Martнnez C., Gonzбlez-Castro A., Vicario M., Santos J. Cellular and molecular basis of intestinal barrier dysfunction in the irritable bowel syndrome // Gut Liver. 2012 Jul; 6(3): 305-15.
2. Turner J.R. Intestinal mucosal barrier function in health and disease // Nat. Rev. Immunol. 2009; 9: 799-809.
3. Garrett W.S., Gordon J.I., Glimcher L.H. Homeostasis and inflammation in the intestine // Cell. 2010; 140: 859-870.
4. Dunlop S.P., Hebden J., Campbell E. et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes // Am. J. Gastroenterol. 2006; 101: 1288-1294.
5. Zhou Q., Zhang B., Verne G.N. Intestinal membrane permeability and hypersensitivity in the irritable bowel syndrome // Pain. 2009; 146: 41-46.
6. Spiller R.C., Jenkins D., Thornley J.P. et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome // Gut. 2000; 47: 804-811.
7. Marshall J.K., Thabane M., Garg A.X. et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario // Aliment. Pharmacol. Ther. 2004; 20: 1317-1322.
8. Mujagic Z., Ludidi S., Keszthelyi D. Small intestinal permeability is increased in diarrhoea predominant IBS, while alterations in gastroduodenal permeability in all IBS subtypes are largely attributable to confounders // Aliment. Pharmacol. Ther. 2014 Aug; 40(3): 288-97.
9. Cheng P., Yao J., Wang C., Zhang L., Kong W. Molecular and cellular mechanisms of tight junction dysfunction in the irritable bowel syndrome // Mol. Med. Rep. 2015; 12(3): 3257-3264.
10. Kong W.M., Gong J., Dong L., Xu J.R. Changes of tight junction claudin-1, -3, -4 protein expression in the intestinal mucosa in patients with irritable bowel syndrome // Nan. Fang. Yi Ke Da Xue Xue Bao. 2007 Sep; 27(9): 1345-7.
11. Martínez C., Vicario M., Ramos L. et al. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations // Am. J. Gastroenterol. 2012 May; 107(5): 736-46.
12. Peters S.A., Edogawa S., Sundt W.J. et al. Constipation-predominant irritable bowel syndrome females have normal colonic barrier and secretory function // Am. J. Gastroenterol. 2017; 112(6): 913-923.
13. Bertiaux-Vandaële N., Youmba S.B., Belmonte L. et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype // Am. J. Gastroenterol. 2011 Dec; 106(12): 2165-73.

/64-1.jpg)
/65-1.jpg)
/65-2.jpg)