Журнал «Почки» Том 8, №3, 2019
Вернуться к номеру
Добовий профіль артеріального тиску в дітей із хронічним пієлонефритом та хронічною хворобою нирок I–III стадій
Авторы: L.I. Vakulenko
State Institution “Dnipropetrovsk Medical Academy of the Ministry of Health of Ukraine”, Dnipro, Ukraine
Рубрики: Нефрология
Разделы: Клинические исследования
Версия для печати
Актуальність. У веденні пацієнтів із хронічною хворобою нирок (ХХН) важливе значення як для зниження традиційного серцево-судинного ризику, так і для збереження залишкової функції нирок протягом тривалого часу має контроль артеріального тиску (АТ). Мета роботи: вивчити особливості добового профілю артеріального тиску в дітей із хронічним пієлонефритом (ХПН) і І–ІІІ стадією ХХН. Матеріали та методи. Обстежені 94 дитини віком від 6 до 17 років із хронічним пієлонефритом поза загостренням і з ХХН I–III стадії. Проводили добове моніторування АТ із наступною математичною обробкою результатів. Результати. При порівняльному аналізі показників АТ, отриманих при разовому вимірюванні та при проведенні ДМАТ, виявлено їх розбіжність у 25,5 % випадків. За даними ДМАТ, у дітей з ХПН в цілому підвищений АТ реєструвався у 22,3 % хворих, АГ — у 34,0 %. Встановлено, що зі зниженням ниркових функцій (легкий або помірний ступінь ХХН) поступово збільшувалась відносна кількість хворих зі стабільною та лабільною АГ. Аналіз ступеня нічного зниження АТ у пацієнтів із ХПН виявив поступове зменшення відносної кількості пацієнтів з оптимальним рівнем зниження як систолічного АТ (від 61,7 % при ХХН І ст. до 47,1 % при ХХН ІІІ ст.), так і діастолічного АТ (53,2 і 11,8 % відповідно; р = 0,0049). Серед хворих із ХХН ІІІ стадії реєстрували пацієнтів night-peakers, які мали стійке підвищення систолічного (11,8 %) та діастолічного АТ (29,4 %) вночі. Висновки. Під час прогресування ХХН серед дітей із ХПН зростає кількість пацієнтів із підвищеним АТ та АГ. Характерними ознаками АГ у цієї категорії пацієнтів є суттєвіший внесок діастолічної складової та стійке підвищення нічного АТ.
Актуальность. В ведении пациентов с хронической болезнью почек (ХБП) важное значение как для снижения традиционного сердечно-сосудистого риска, так и для сохранения остаточной функции почек в течение длительного времени имеет контроль артериального давления (АД). Цель работы: изучить особенности суточного профиля АД у детей с хроническим пиелонефритом (ХПН) с І–ІІІ стадией ХБП. Материалы и методы. Обследованы 94 ребенка в возрасте от 6 до 17 лет с хроническим пиелонефритом вне обострения и ХБП I–III стадии. Проводилось суточное мониторирование артериального давления (СМАД) с последующей математической обработкой результатов. Результаты. При сравнительном анализе показателей АД, полученных при разовом измерении и при проведении СМАД, выявлено их расхождение в 25,5 % случаев. По данным СМАД, у детей с ХПН в целом повышенное АД регистрировалось у 22,3 % больных, АГ — у 34,0 %. Установлено, что со снижением функции почек (легкая или умеренная степень ХБП) постепенно увеличивалось относительное количество больных со стабильной и лабильной артериальной гипертензией (АГ). Анализ степени ночного снижения АД у пациентов с ХПН показал постепенное уменьшение относительного количества пациентов с оптимальным уровнем снижения как систолического АД (от 61,7 % при ХБП I стадии до 47,1 % при ХБП III стадии), так и диастолического АД (53,2 и 11,8 % соответственно; р = 0,0049). Среди больных с ХБП III стадии регистрировались пациенты night peakers, которые имели устойчивое повышение систолического (11,8 %) и диастолического АД (29,4 %) ночью. Выводы. Во время прогрессирования ХБП среди детей с ХПН увеличивается количество пациентов с повышенным АД и АГ. Характерными признаками АГ у этой категории пациентов являются существенный вклад диастолической составляющей и устойчивое повышение ночного АД.
Background. Blood pressure (BP) monitoring is important for the management of patients with chronic kidney disease (CKD), both for the conventional cardiovascular risk reduction and long-term preservation of kidney function. The purpose was to study the features of 24-hour blood pressure profile in children with chronic pyelonephritis (CPN) and CKD stages І–ІІІ. Materials and methods. A total of 94 patients aged from 6 to 17 years with chronic pyelonephritis in remission and CKD stages І–ІІІ were examined. 24-hour ambulatory blood pressure monitoring (ABPM) was carried out followed by mathematical processing. Results. Thus, a comparative analysis of the systolic (SBP) and diastolic blood pressure (DBP) indicators obtained within a single BP measurement and during 24-hour ABPM showed their differences in 25.5 % of cases. According to ABPM findings, 22.3 % of CKD children demonstrated elevated BP and 34.0 % — arterial hypertension (АН). It was found that the relative number of patients with sustained and labile AH gradually increased with a decrease in renal functions (mild-to-moderate CKD progression). The analysis of night-time BP dipping degree in patients with CKD revealed a gradual decrease in the relative number of patients with optimal BP dipping for both SBP (from 61.7 % in CKD stage I to 47.1 % in CKD stage III) and DBP (53.2 and 11.8 %, respectively; р = 0.0049). Night-peakers with night-time stable elevation of SBP (11.8 %) and DBP (29.4 %) were the patients with CKD stage III. Conclusions. The number of hypertensive patients increases among CPN children during CKD progression. AH in children with progressive nephropathy is characterized by a greater contribution from DBP and stable elevation of night-time BP.
артеріальний тиск; добове моніторування артеріального тиску; діти; хронічний пієлонефрит; хронічна хвороба нирок
артериальное давление; суточное мониторирование артериального давления; дети; хронический пиелонефрит; хроническая болезнь почек
blood pressure; 24-hour blood pressure profile; children; chronic pyelonephritis; chronic kidney disease
Introduction
Today, pathophysiological mechanisms leading to increased cardiovascular risk in patients with chronic kidney disease (CKD) have not been fully understood, but there is hard evidence of a close link between heart and kidney [1–4]. Interaction between kidney diseases and the cardiovascular system, so called “cardiorenal syndrome” (CRS) [1–3], has been much discussed recently. Cardiorenal syndrome involves a variety of acute and chronic diseases in which the dysfunction of either the heart or kidneys may cause the failure of the other organ [3, 4]. CRS type 4, or chronic renocardiac syndrome, is defined as “chronic kidney pathology leading to heart failure”, refers to the development of cardiovascular system pathology at any stage of CKD [1, 2, 4, 5]. Patients with CKD are particularly prone to developing cardiac dysfunction due to the high prevalence of cardiovascular risk factors in this population, but the contribution of specific risk factors should be taken into account [5]. The most important risk factor for developing renocardiac disorders is increased blood pressure (BP), both in adults and children [1, 4, 6].
A number of studies have shown that high BP plays the role of an independent risk factor for rapid reduction in glomerular filtration rate (GFR) in patients with renal diseases [6–9]. BP monitoring is important for the management of patients with CKD, both for the conventional cardiovascular risk reduction and long-term preservation of kidney function [8]. The parameters of 24-hour ambulatory blood pressure monitoring (ABPM) have more pronounced predictive value for the diagnosis of target organ damage than the indicators determined at a single office/home BP measurement [8, 10].
Normal BP values assessed by a single office measurement cannot rule out masked arterial hypertension (AH), night-time hypertension and other deviations from the norm in children with CKD [8, 10]. 2017 American Academy of Pediatrics Guidance recommends the 24-hour ABPM for children and adolescents with CKD, regardless of routine office BP measurements, at least once a year [8, 11].
The purpose of our work was to determine the features of 24-hour blood pressure profile in children with chronic pyelonephritis (CPN) and chronic kidney disease stages І–ІІІ.
Materials and methods
A total of 94 patients aged from 6 to 17 years (41 boys, 53 girls) with chronic pyelonephritis and CKD stages І–ІІІ were examined. The control group consisted of 78 apparently healthy children of the corresponding age and sex. All the patients received inpatient treatment in the Nephrology Department of SI “Dnipropetrovsk Regional Children’s Clinical Hospital of DRC”. The planned clinical study was approved by the Bioethics Committee of the SI “Dnipropetrovsk Medical Academy of the Ministry of Health of Ukraine” and conducted in accordance with the 1975 version of the Declaration of Helsinki. The criteria for inclusion in the study were: the presence of a voluntary informed consent of a child and his/her parents for study participation; age of patients from 6 years to 17 years 11 months and 29 days; the presence of verified diagnoses of CPN and CKD stages І–ІІІ; the absence of clinical and laboratory signs of CPN exacerbation. The criteria for exclusion of patients from the study were: refusal of a child or his/her parents to participate in the study; the presence of congenital heart disease or other primary cardiac diseases, acute infections, diabetes mellitus, essential or neuroendocrine AH.
Based on the renal functions state, children were divided into respective groups: group 1 — 47 patients with CKD stage I; group 2 — 30 patients with CKD stage II; group 3 — 17 children with CKD stage III.
The office BP measurement was performed three times with an interval of 2–3 minutes after rest for 10–15 minutes beforehand in a comfortable sitting position using a tonometer with an appropriately fitted cuff corresponding to the age and arm circumference.
24-hour ABPM was carried out using the software-hardware system “Cardiotechnica-04-BP-1” (ZAO “INCART”, St. Petersburg, RF). The following quantitative parameters were assessed analyzing the 24-hour ABPM results: average 24-hour BP, mean daytime and mean night-time values of systolic (SBP) and diastolic BP (DBP), mean BP, pulse BP, heart rate, maximum and minimum values of SBP, DBP and heart rate, indexes of SBP and DBP load at daytime and night-time (time rate index), the magnitude and velocity of SBP and DBP morning surge.
Daytime and night-time SBP and DBP variability was determined by the standard deviation of these indicators from the mean value. The degree of nocturnal BP dipping or daily index (the ratio of average daytime BP to average night-time BP values, SBP daily index, DBP daily index) were also calculated. The average 24-hour, mean daytime and mean night-time SBP and DBP values were assessed using percentile tables (percentiles 5, 50, 90, 95) depending on the child’s gender and height.
When evaluating BP, normal BP, elevated BP and hypertension were identified using the 2017 American Academy of Pediatrics Guidance [11]. Sustained AH was diagnosed at an average 24-hour BP level above 95th percentile with the index of BP load of more than 50 %. Labile AH was determined if the index of BP load ranged from 25 to 50 %, but an average 24-hour BP level was below 95th percentile.
By the value of BP daily index, 4 variants were distinguished: dippers — the daily index 10–22 %; non-dippers — daily index 0–10 %; over-dippers — daily index over 22 %; night-peakers — daily index less than 0 %.
The results were statistically analyzed using the Statistica 8.0 package. The Shapiro-Wilk normality test was used to check the normal distribution of variables according to the Gaussian distribution model. Given the non-Gaussian distribution in most of the samples, the results were presented as median (Me) and interquartile range (Q25; Q75). The mean values between groups were compared using the Mann-Whitney test. A value of p < 0.05 was considered statistically significant.
Results
According to the results of a single BP measurement, elevated BP was diagnosed in 9.6 % and AH in 21.3 % of patients, while 24-hour ABPM revealed elevated BP in 22.3 % and AH in 34.0 % of patients. Thus, a comparative analysis of the SBP and DBP indicators, obtained at a single BP measurement and during 24-hour ABPM, showed their differences in 25.5 % of cases. Masked AH was registered in 12.8 % of patients.
The structure of BP in the examined children with CPN and CKD is presented in Table 1.
Analysis of the main integrated indicators determined during 24-hour ABPM in children with CPN and CKD found the relative number of AH patients gradually increased with a decrease in renal functions (mild-to-moderate CKD progression).
The maximum pathological changes occurred in the group of children with CPN and CKD stage III, as AH was diagnosed in more than half of the patients — 58.8 %. AH stage I prevailed in the structure of AH (21.3, 26.7 and 41.2 % in CKD stage І, ІІ and ІІІ, respectively).
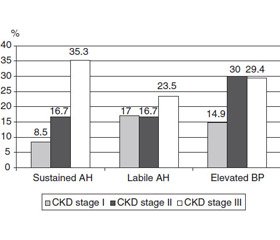
The relative number of patients with stable and labile AH gradually increased with CKD progression (Fig. 1).
In the overall structure of hypertension, labile hypertension prevailed in CPN patients (53.1 %), stable hypertension was detected somewhat less frequently — in 46.9 % of children. The proportion of hypertensive patients (stable AH + labile AH) in the group of children with CKD stage III was statistically significantly higher than in patients with CKD stage I (58.8 and 25.5 %, respectively; p = 0.0172) and in those with CKD II stage (58.8 and 33.4 %, respectively; p = 0.0290).
The analysis of night-time BP dipping degree in CKD patients revealed a gradual decrease in the relative number of patients with optimal BP dipping (dippers) in CKD mild-to-moderate progression for both SBP (from 61.7 % in CKD stage I to 47.1 % in CKD stage III) and DBP (53.2 and 11.8 %, respectively; p = 0.0049) (Fig. 2).
By contrast, the relative number of patients with an inadequate night-time BP dipping (non-dippers) increased in reference to the indicators of SBP (from 29.8 % in CKD stage I to 41.1 % in CKD stage III) and DBP (25.5 and 58.8 %, respectively; p = 0.0172) (Fig. 3).
Night-peakers with night-time stable elevation of SBP (11.8 %) and DBP (29.4 %) were detected among patients with CKD stage III.
The study of BP morning pattern, namely the magnitude and velocity of SBP and DBP morning surge, found a number of features (Tables 2, 3).
The median of SBP morning surge did not exceed the normative value of 56.5 mm Hg in all groups of the examined children. The median velocity of DBP morning surge was elevated in all examined groups, while the median of SBP morning surge was above the norm only in children with CKD stage I.
The detailed analysis found that the relative number of children with increased magnitude of SBP morning surge in CKD stage III was statistically significantly higher than that in case of CKD stage I (29.4 and 4.2 %, respectively; p = 0.0057) and CKD stage ІІ (29.4 and 3.3 %, respectively; p = 0.0126) (Table 3).
The proportion of children with increased velocity of SBP and DBP morning surge did not differ statistically significantly between examined groups, but the relative number of patients with increased velocity of DBP morning surge was significantly higher in CKD stage II (63.3 and 33.3 %, respectively; p = 0.0256) and CKD stage ІІІ (58.8 and 23.5 %, respectively; p = 0.0465).
Discussion
AH is considered to be one of the main factors contributing to the progression of CKD, increasing the risk of cardiovascular complications and suppressing neurocognitive functions [12, 13]. Our results of BP studies suggest that the number of hypertensive patients increases in CKD progression towards the terminal stage of chronic renal failure. These results are supported by the data of the CKiD study, which determined an increase in the number of children with AH and cardiovascular complications due to reduced kidney function [14].
Comparative analysis of 24-hour ABPM and office BP measurement results shows that a statistically significantly greater number of patients were diagnosed with AH (34.0 and 21.3 %, respectively; p = 0.0474) and elevated BP (22.3 and 9.6 %, respectively; p = 0.0385) assessed by 24-hour ABPM. The data obtained coincide with the results of other researchers. Thus, when comparing the results of office BP measurement and 24-hour ABPM in 359 children with CKD and GFR at least 30 ml/min, the latter method revealed a greater relative number of patients with both elevated BP and AH [10].
A detailed analysis of 24-hour BP profile in children with CPN and CKD demonstrates DBP and night-time stably elevated BP to be great contributors to AH. Previous studies also show that masked AH is diagnosed mainly by DBP [15] and the detection of isolated night-time BP elevation [16]. These data can be an argument to conclude that BP normal level assessed by a single office measurement does not allow ruling out masked AH in CKD children. Therefore, it is now recommended to perform 24-hour ABPM for all CKD children regardless of BP level assessed by a single office BP measurement [17–19].
The analysis of night-time BP dipping degree in patients with CKD revealed a gradual decrease in the relative number of patients with optimal BP dipping (dippers) in CKD progression and thus, an increase in the number of non-dipper patients. Taking into consideration that non-dipping BP pattern is associated with suppression of parasympathetic nervous system activity and an increase in sympathetic nervous system activity throughout the night, it appears that hypersympathicotonia significantly influence not only 24-hour BP profile and AH development, but also CKD progression [20]. In the view of most clinicians, the non-dipping circadian rhythm of BP in CKD patients is closely linked to cardiovascular complications, particularly, left ventricular hypertrophy [21].
Although statistically significant between-groups differences in indices of BP morning surge were not obtained by a majority of comparisons, the fact of an increase in magnitude and velocity of BP morning surge in CKD children with reduced renal function deserves attention. It should be noted that the morning BP surge is mediated by rapid sympathetic nervous system activation with the development of vasoconstriction [7, 22]. Also, in the morning, the endothelial function decreases, and the level of thrombogenic factors increases [23, 24]. All these processes can result in cardiovascular events in CKD patients [25]. So, the presence of increased and rapid morning BP surge in CKD patients considers them not only as a risk group for future cardiovascular events but also indicates an increased risk of target organ damage.
Conclusions
The number of hypertensive patients increases among CPN children at the stage of CKD progression. AH in children with progressive nephropathy is characterized by a greater contribution from DBP and stable elevation of night-time BP. Increased night-time BP can be considered as an indicator of AH stability and an additional risk factor for cardiovascular complications in this category of patients. Indicators of the 24-hour ABPM, especially which are tied to the circadian rhythm of BP, demonstrate a significant role of the autonomic nervous system dysfunction in the development and progression of CKD as well as cardiovascular pathology.
Conflicts of interests. The author declares the absence of any conflicts of interests with respect to the research, authorship, and publication of this article.
Peer-viewers: head of the Pediatric Department 2 of Kharkiv National Medical University, MD, PhD, Professor Makieieva N.I.; head of the Department of Family Medicine of SI “Dnipropetrovsk Medical Academy of MH of Ukraine”, MD, PhD, Professor Vysochyna I.L.
1. Di Lullo L. Hypertension, type IV cardiorenal syndrome and chronic kidney disease: Pathophysiological and therapeutical approach. World. J. Hypertens. 2017. № 7 (1). Р. 10-18. doi: 10.5494/wjh.v7.i1.10.
2. Edmonston D., Morris J.D., Middleton J.P. Working Toward an Improved Understanding of Chronic Cardiorenal Syndrome Type 4. Advances in Chronic Kidney Disease. 2018. № 25(5). Р. 454-467. doi: 10.1053/j.ackd.2018.08.010.
3. Rangaswami J., Bhalla V., Blair J.E.A., Chang T.I., Costa S. L.K.L. et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement from the American Heart Association. Circulation. 2019. № 139(16). e840-e878. doi: 10.1161/CIR.0000000000000664.
4. Kaddourah A., Goldstein S.L. Heart Failure in the Child and Young Adult. Chapter 31. Childhood Cardiorenal Syndrome. Academic Press. 2018. doi: 10.1016/C2014-0-01738-8
5. Pinheiro da Silva A.L., da Silva M.J. Type 4 cardiorenal syndrome. Rev. Port. Cardiol. 2016. № 35(11). Р. 601-616. doi: 10.1016/j.repc.2016.06.007.
6. Vidi S.R. Role of hypertension in progression of chronic kidney disease in children. Curr. Opin. Pediatr. 2018. № 30. Р. 247-251. doi: 10.1097/MOP.0000000000000595.
7. Bilo G., Grillo A., Guida V., Parati G. Morning blood pressure surge: pathophysiology, clinical relevance and therapeutic aspects. Integr. Blood Press. Control. 2018. № 11. Р. 47-56. doi: 10.2147/IBPC.S130277.
8. Gabriele M.M., Nogueira P.C.K. Management of Hypertension in CAKUT: Protective Factor for CKD. Front. Pediatr. 2019. № 7. Р. 222. doi: 10.3389/fped.2019.00222.
9. Waraby B.A., Abraham A.G., Schwartz G.J. et al. Predictors of rapid progression of glomerular and nonglomerular kidney disease in children and adolescents: the chronic kidney disease in children (CKiD) cohort. Am. J. Kidney Dis. 2015. № 65. Р. 878-788. doi: 10.1053/j.ajkd.2015.01.008.
10. Аксенова М.Е., Конькова Н.Е., Лепаева Т.В., Кырганова Т.А., Длин В.В. Диагностическое значение уровня разового артериального давления для выявления скрытой артериальной гипертензии у детей с хроническими болезнями почек. Российский вестник перинатологии и педиатрии. 2018. № 62 (2). С. 54-59.
11. Flynn J.T., Kaelber D.C., Baker-Smith C.M. et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017. № 140. Р. 1-72. e20171904. doi: 10.1542/peds.2017-1904.
12. Hamrahian S.M., Falkner В. Hypertension in Chronic Kidney Disease. Adv. Exp. Med. Biol. 2017. № 956. Р. 307-325. doi: 10.1007/5584_2016_84.
13. Lande M.B., Mendley S.R., Matheson M.B. et al. Association of blood pressure variability and neurocognition in children with chronic kidney disease. Pediatr. Nephrol. 2016. № 31. Р. 2137-2144. doi: 10.1007/s00467-016-3425-2.
14. Barletta G.M., Flynn J., Mitsnefes M. et al. Heart rate and blood pressure variability in children with chronic kidney disease: a report from the CKiD study. Hypertension. 2018. № 71 (3). Р. 444-450. doi: 10.1007/s00467-013-2737-8.
15. Cilsal E., Koc A.S. Renal resistive index significantly increased in hypertensive children and it is independently related to the pulse pressure and left ventricular mass index. Clin. Exp. Hypertens. 2018. № 4. Р. 1-8. doi: 10.1080/10641963.2018.1523920.
16. Mitsnefes M.M., Piierce C., Flynn J. et al. For the CKiD study group. Can office blood pressure readings predict masked hypertension? Pediatr. Nephrol. 2016. № 31. Р. 163-166. doi: 10.1007/s00467-015-3212-5.
17. Andrade H., Pires A., Noronha N. et al. Importance of ambulatory blood pressure monitoring in the diagnosis and prognosis of pediatric hypertension. Rev. Port. Cardiol. 2018. № 37 (9). Р. 783-789. doi: 10.1016/j.repc.2017.09.026.
18. Gupta D., Chaturvedi S., Chandy S., Agarwal I. Role of 24-h ambulatory blood pressure monitoring in children with chronic kidney disease Indian. J. Nephrol. 2015. № 25 (6). Р. 355-361. doi: 10.4103/0971-4065.148305.
19. Peterson C.G., Miyashita Y. The Use of Ambulatory Blood Pressure Monitoring as Standard of Care in Pediatrics. Front. Pediatr. 2017. № 5. Р. 153. doi: 10.3389/fped.2017.00153.
20. Fedecostante M., Spannella F., Cola G., Espinosa E., Dessì-Fulgheri P., Sarzani R. Chronic kidney disease is characterized by “double trouble” higher pulse pressure plus night-time systolic blood pressure and more severe cardiac damage. PLoS One. 2014. № 9 (1). e86155. doi: 10.1371/journal.pone.0086155.
21. Che X., Mou S., Zhang W. et al. The impact of non-dipper circadian rhythm of blood pressure on left ventricular hypertrophy in patients with non-dialysis chronic kidney disease. Acta Cardiol. 2017. № 72 (2). Р. 149-155. doi: 10.1080/00015385.2017.1291133.
22. Johnson A.W., Hissen A.L., Macefield V.G., Brown R., Taylor C.E. Magnitude of Morning Surge in Blood Pressure Is Associated with Sympathetic but Not Cardiac Baroreflex Sensitivity. Front Neurosci. 2016. №10. Р. 412. doi: 10.3389/fnins.2016.00412.
23. Kıvrak A., Özbiçer S., Kalkan G.Y., Gür M. Morning blood pressure surge and arterial stiffness in newly diagnosed hypertensive patients. Blood Press. 2017. № 26 (3). Р. 181-190. doi: 10.1080/08037051.2017.1278678.
24. Mahfouz R.A., Goda M., Galal I., Ghareb M.S. Association of morning blood pressure surge with carotid intima-media thickness and cardiac dysfunction in patients with cardiac syndrome-X. Blood Press. 2018. № 27 (5). Р. 297-303. doi: 10.1080/08037051.2018.1476056.
25. Sheppard J.P., Hodgkinson J., Riley R., Martin U., Bayliss S., McManus R.J. Prognostic Significance of the Morning Blood Pressure Surge in Clinical Practice: A Systematic Review. Am. J. Hypertens. 2015. № 28 (1). Р. 30-41. doi: 10.1093/ajh/hpu104.

/13-1.jpg)
/13-2.jpg)
/14-1.jpg)