Резюме
Мета. Хвороба Хашимото (ХХ) є автоімунним захворюванням, у патогенезі якого відіграють роль як генетичні, так і зовнішні чинники. До останніх належить надходження йоду з їжею. Виведення йоду з сечею при збалансованому харчуванні рівною мірою відповідає його надходженню ззовні. Існує думка, згідно з якою висока концентрація йоду в сечі може бути пов’язана з автоімунним захворюванням щитоподібної залози. Мета нашої роботи — вивчити, чи підвищений рівень йоду в сечі у пацієнтів з ХХ порівняно з загальною популяцією. Матеріали та методи. Дослідження включало 64 пацієнтів з ХХ і 39 здорових добровольців. У всіх пацієнтів вивчали такі показники: вік, стать, рівень у сироватці крові вільного трийодтироніну (сТ3), вільного тироксину (сТ4), тиреотропного гормона (ТТГ), антитіл до тиреоїдної пероксидази (АТ-ТПО), антитіл до тиреоглобуліну (АТГ), а також концентрацію йоду в сечі. Було проведено ультрасонографію щитоподібної залози. Концентрацію йоду в сечі визначали з використанням реакції Санделла — Колтхофа. Результати. У нашому дослідженні не було виявлено вірогідного зв’язку між загальним обсягом щитоподібної залози і концентрацією йоду в сечі (p > 0,05). Також не було виявлено вірогідної різниці між рівнями сТ3, сТ4, АТ-ТПО, АТГ і об’ємом щитоподібної залози в осіб із концентрацією йоду в сечі вище і нижче від 100 мкг/л. Різниця сягала вірогідного рівня тільки при порівнянні показників ТТГ (р = 0,04). Не було виявлено різниці між загальним обсягом щитоподібної залози. У нашому дослідженні не було виявлено зв’язку між ХХ і рівнем йоду в сечі. З огляду на те, що поширеність ХХ у нашій країні відповідає даним, зазначеним у літературі, постає питання про роль йоду, що надходить з їжею, в патогенезі захворювання. Висновки. У йододефіцитних регіонах, до яких належить зокрема й наша країна, необхідно вивчати зв’язок між рівнем йоду в сечі та ХХ.
Цель. Болезнь Хашимото (БХ) является аутоиммунным заболеванием, в патогенезе которого играют роль как генетические, так и внешние факторы. К последним относится поступление йода с пищей. Выведение йода с мочой при сбалансированном питании в равной мере соответствует его поступлению извне. Существует мнение, согласно которому высокая концентрация йода в моче может быть связана с аутоиммунным заболеванием щитовидной железы. Цель нашей работы — изучить, повышен ли уровень йода в моче у пациентов с БХ по сравнению с общей популяцией. Материалы и методы. Исследование включало 64 пациентов с БХ и 39 здоровых добровольцев. У всех пациентов изучали следующие показатели: возраст, пол, уровень в сыворотке крови свободного трийодтиронина (сТ3), свободного тироксина (сТ4), тиреотропного гормона (ТТГ), антител к тиреоидной пероксидазе (АТ-ТПО), антител к тиреоглобулину (АТГ), а также концентрацию йода в моче. Была проведена ультрасонография щитовидной железы. Концентрацию йода в моче определяли с использованием реакции Санделла — Колтхофа. Результаты. В нашем исследовании не было выявлено достоверной связи между общим объемом щитовидной железы и концентрацией йода в моче (p > 0,05). Также не было обнаружено достоверной разницы между уровнями сТ3, сТ4, АТ-ТПО, АТГ и объемом щитовидной железы у лиц с концентрацией йода в моче выше и ниже 100 мкг/л. Разница достигала достоверного уровня только при сравнении показателей ТТГ (р = 0,04). Не было выявлено разницы между общим объемом щитовидной железы. В нашем исследовании не было обнаружено связи между БХ и уровнем йода в моче. Ввиду того, что распространенность БХ в нашей стране соответствует данным, указанным в литературе, встает вопрос о роли йода, поступающего с пищей, в патогенезе заболевания. Выводы. В йододефицитных регионах, к которым относится в том числе и наша страна, необходимо изучать связь между уровнем йода в моче и БХ.
Background. Hashimoto’s thyroiditis (HT) is an autoimmune disease which genetic and environmental factors play a role. One of the environmental risk factors is dietary iodine intake. Urinary iodine excretion in balanced diet is equally acceptable with received iodine. It’s thought that high urinary iodine excretion was associated with autoimmune thyroid diseases. We purposed to investigate whether urinary iodine level is higher in patients with HT than population. Materials and methods. 64 new patients with HT and 39 healthy volunteers were included. Age, gender, serum free-triiodothyronine (fT3), free-thyroxine (fT4), thyroid-stimulating hormone (TSH), anti thyroid peroxidase antibody (anti-TPO), anti thyroglobulin antibody (anti-TG) and urinary iodine concentration (UIC) were evaluated. Thyroid ultrasonography was performed. UIC were measured by Sandell-Kolthoff method. Results. There was no significant relationship was found between total thyroid volume and UIC in our study (p > 0.05). There was no significant difference between the fT3, fT4, anti-TPO, anti-TG and thyroid volume values of the individuals with urinary iodine levels below and above 100 μg/L. The difference between two subgroups was found to be significant only when TSH values were compared (p = 0.04). There was no significant difference between total thyroid volumes. No relation was found between HT and urine iodine levels in our study. The fact that the prevalence of HT in our country is similar to the literature makes us question the role of dietary iodine in the etiology. Conclusions. At the iodine-deficient regions such as our country relationship between urinary iodine excretion and HT needs to be investigated.
Introduction
Hashimoto’s thyroiditis (HT) is a disease which genetic and environmental factors play a role. It is an autoimmune disease with antibody-mediated chronic destruction of the thyroid gland tissue. It is the most common cause of hypothyroidism and goitre in regions without endemic iodine deficiency in the world. HT is frequently seen in 30–50 years of age, but is more common in older ages. It is seen in female sex 5–8 times more than men [1]. The prevalence is reported as 4.6 % [1].
For the first time in 1912, HT was defined by Dr. Hakaru Hashimoto [2]. The most common finding in the beginning of the disease is the presence of goitre with euthyroidism or hypothyroidism. 75 % of patients have euthyroid goiter. Therefore, the disease can be asymptomatic and can be diagnosed incidentally. In the presence of suspicion of disease; thyroid autoantibodies, thyroid function tests and ultrasonographic findings help to support the diagnosis [3]. HT is more common in individuals who is genetically predisposed. HT is associated with various polymorphisms in the human leukocyte antigen (HLA) gene. The prevalence of some HLA types has increased compared to the general population [4]. 30–40 % of patients have family history [4].
Environmental risk factors for HT are not well defined. Environmental factors likely to play a role in etiology; bacterial and viral infections, advanced age, radiation exposure, cytokine therapy, pregnancy and dietary iodine intake [5].
Iodine, one of the risk factors, is an essential component for the synthesis of thyroid hormones. Like other trace elements, it is essential. The thyroid gland receives iodine from the extracellular compartment by means of carrier proteins in the cell membrane. Approximately 85–90 % of the iodine taken is excreted in the urine. Urinary iodine excretion in balanced diet is equally acceptable with received iodine [6]. According to the World Health Organization, the average iodine concentration in urine should be above 100 μg/L in areas with adequate iodine. Urinary iodine concentration (UIC) above 200 μg/L indicates iodine excess [7].
Iodine levels in urine may vary between individuals and may change at different times in the same individual during the day. However, although these changes may be important for individuals, they can be ignored in the presence of multiple samples in community evaluation [8]. Although there are differences in hydration status among individuals, there is a good correlation between 24-hour urine samples and spot urine samples [8].
After increased dietary intake of iodine in endemic goiter regions, serum thyroid autoantibody concentrations increased [9]. In a rat experiment in the literature shown that excess iodine intake directly affects thyroglobulin molecules. And immunogenic high iodized thyroglobulin molecules lead to HT [9].
The purpose was to investigate whether urinary iodine level is higher in patients with HT than population.
Materials and methods
The study protocol was approved by the ethics committee of the Ege University Medical School. In this cross-sectional study of 103 patients from the Izmir region conducted between June 2016 and September 2017, 64 newly diagnosed cases of Hashimoto’s Thyroiditis and 39 healthy volunteers were assessed.
Participant inclusion criteria were as follows:
1. Resident of locality for > 1 year.
2. Age 18–75 range.
Participant exclusion criteria were as follows:
1. Female participants, pregnant, breastfeeding, or within 1 year after childbirth.
2. Took thyroid medication in the past 15 days.
3. Was a hospital inpatient or seriously ill during the previous 4 weeks.
4. Surgery in the past 6 months.
5. On a high-iodine diet or consumed seafood including kelp, sea fish, crab, shrimp, and shellfish in the past 3 days.
Hashimoto’s thyroiditis were diagnosed in the presence one of clinical and laboratory criteria:
1. Diffuse swelling of the thyroid gland without any other cause (such as Graves’ disease).
2. Positive anti-TPO and/or Positive anti-TG and/or Lymphocytic infiltration in the thyroid gland confirmed with cytological examination and/or evidence of a ultrasound hypoechoic patter
3. Laboratory tests consistent with hypothyroidism, an elevated serum TSH with low thyroid hormone (Free thyroxine) levels.
Age, gender and family history were evaluated in all subjects. Serum fT3, fT4, TSH, anti-TPO, anti-TG concentrations are assayed with Elecsys Electrochemiluminescence (ECLIA) kit by Cobas e 801 systems. For TSH method, the results of the intra assay and inter assay %CV values are as follows with 3 different concentrations of human serum (0.034, 0.91, and 3.96 μlU/mL). While intraassay %CV are 8.2, 2.1, and 1.8 interassay %CV of the method are 8.7, 3.3 and 3.6 respectively for the given concentrations. For fT3 assay, the results of the intra assay and inter assay %CV values are as follows with 4 different concentrations of human serum (0.095, 0.209, 0.448 and 1.75 ng/dL). While intraassay %CV are 7.6, 3.5, 2.1 and 1.7 interassay %CV of the method are 8.3, 3.8, 2.5 and 1.8 respectively for the given concentrations. For fT4 assays the results of the intra assay and inter assay %CV values are as follows with 3 different mean concentrations of human serum (1.25, 10.02 and 42.02 pg/mL). While intraassay %CV are 6.4, 2.4 and 1.5, interassay %CV of the method are 8.1, 2.6, and 2.5 respectively for the given concentrations.
Laboratory reference ranges for various parameters were as follows: (i) TSH, 0.35–5.50 µIU/mL; (ii) fT4, 0.89–1.76 ng/dL; (iii) fT3, 2.3–4.2 pg/mL, (iv) , anti-TPO, < 60 IU/ml; and (v) anti-TG, < 60 IU/ml. Thyroid ultrasonography was performed by the same physician and total thyroid volume was determined. Spot urine samples were taken from participants between 8:00–12:00 in the morning. Concentrated HCl was added to the urine samples with 1 drop to 2.5 ml and stored at –80 °C until analysis. On the day of analysis, samples were centrifuged for three minutes after dissolution at room temperature and iodine measurements were performed. The urinary iodine method depends on the manual digestion with ammonium persulfate followed by the calorimetrically determination of the Sandell-Kolthoff reaction [10] using 96 multiwell plates and an absorbance microplate reader at 405 nm. For urinary iodine analysis the results of intraassay and inter assay %CV values for 5 different concentrations (5, 10, 40, 160 and 300 μg/L) were calculated. While intraassay %CV values are 7.6, 4.2, 2.7, 3.1, and 4.5, interassay %CV values of the method are 8.5, 5.8, 4.4, 4.1 and 5.4 respectively for the given concentrations. Cut off levels differ according to different target groups. For adults (but not pregnant or lactating) < 100μg/L is considered insufficient iodine intake. 100–199μg/L is adequate and > 200μg/L is considered above requirement according to EQUIP Booklet — CDC. Iodine status was classified according to the WHO/UNICEF/International Council for Control of Iodine Deficiency Disorders guidelines: (i) severely insufficient, < 20 μg/l; (ii) mild-to-moderate insufficient, 20–99 μg/l; (iii) sufficient, 100–199 μg/l; (iv) more than adequate, 200–299 μg/l; and (v) excessive, ≥ 300 μg/l [7].
Statistical analysis. Statistical analysis of the data was done by using SPSS 22.0™ package program. For intergroup comparison cross-table comparison and chi-square test were used in categorical variables. Two groups were compared with t-test in independent groups in numerical variables. Data were presented as arithmetic mean (standard deviation) for numerical variables and as number and percentage for categorical variables. The Mann-Whitney-U test was used to compare the two groups for non-normally distributed numerical variables. Statistical significance level was accepted as p < 0.05.
Results
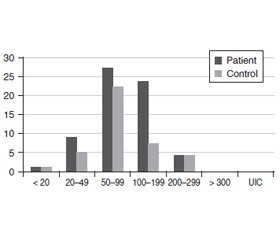
In our study, there was no significant difference in urinary iodine levels between the groups. The mean (SD) UIC was 102.31 (54.63) μg/L in the patient group and 91.88 (55.74) μg/L in the control group. The distribution of UIC in the subgroups is shared in Table 1 and figure 1.
There were 50 women and 14 men in the patient group and 22 women and 17 men in the control group. The mean (SD) age of the patient group was 43.91 (15.89) years and 37.69 (12.29) years in the control group. 15 (23.4 %) patients had a family history in the patient group. There was no significant difference in family history of autoimmune thyroid disease in subgroups. HT group was classified according to thyroid dysfunction. 47 patients were euthyroid (73 %), 7 patients were overt hypothyroid (11 %), 3 patients were subclinical hypothyroid (5 %), 2 patients were subclinical hyperthyroid (3 %) and 5 patients were overt hyperthyroid (8 %). There was no significant relationship between urine iodine levels among the subgroups.
The mean (SD) total thyroid volume was 12 (5.16) ml in the patient group and 10.33 (4.86) ml in the control group. There was also no significant difference between total thyroid volumes.
There was no significant difference between the fT3, fT4, anti-TPO, anti-TG and thyroid volume values of the individuals with urinary iodine levels below and above 100 μg/L. The difference between two subgroups was found to be significant only when TSH values were compared (p = 0.04). Serum TSH levels increased when UIC increased.
Thyroid function tests, UIC and thyroid volume values of the patient and control groups were shared in Table 2.
Discussion
It’s thought that high urinary iodine excretion in population was associated with autoimmune thyroid diseases [11]. Serum antithyroid antibody concentrations increased after dietary iodine intake in endemic goiter regions [11]. The pathogenesis of dietary iodine contribution to thyroid autoimmunity is unknown. Various hypotheses have been proposed about this subject [11].
To understand the role of urinary iodine excretion in thyroid diseases, in a study conducted in a large group of patients, pathology results were compared with UIC values in patients who underwent thyroid surgery for any reason; high UIC is associated with increased prevalence of HT [12]. In many similar studies, autoimmune thyroiditis prevalence and increased autoantibody positivity have been reported in individuals with excessive iodine intake [13–15]. In a meta-analysis of a series of studies, moderate iodine excess (median urinary iodine excretion > 220 μg/L) was associated with a high prevalence of hypothyroidism in elderly patients [16]. Xu et al. in their research using cell culture, thyroid follicular cells with in vitro excess iodine have been shown to contribute to the suppression of autophagy activity and apoptosis [17]. In a study conducted in Northern Morocco, there was a temporary enhancement in the prevalence of detectable antibodies after than increased levels of UIC, but these values returned to the reference values within 1 year. None of the patients developed clinical or ultrasonographic findings of thyroid autoimmune disease [18].
M.F. Erdoğan et al in their research to determine the iodine status in Turkey; Aegean region is assessed as adequate iodine region according to UIC [19]. In another study, the volume of thyroid and UIC was examined in the Aegean region; indicated that ranging from mild to severe levels of iodine deficiency is still present in Turkey’s Aegean coast [20]. Iodine status of the population in Turkey after iodisation of salt and chronic effects of high iodine intake are not well known. A study in our country investigated the effects of iodine on HT, no significant relationship was found between UIC and HT [21].
Urinary iodine levels in our study show that our region is at the limit of mild to moderate iodine deficiency. In our study, no significant difference was found between the patient and control group according to urine iodine levels. In contrast to other studies in the literature, there is no direct relationship between HT and iodine intake in our region. Erdoğan et al. were found the prevalence of HT 3.9 % in a large patient group at Turkey’s Aegean region [22]. The fact that the prevalence of HT in our country is similar to the literature makes us question the role of dietary iodine in the etiology. At the iodine-deficient regions such as our country need to be investigated to relationship between UIC and HT.
When the patient and control groups were evaluated together, no significant relationship was found between total thyroid volume and UIC in our study. The fact that UIC values are close to the normal limits in both groups explains this situation. Similar studies investigating the relationship between thyroid volume and UIC in literature have shown the role of iodine deficiency or excess iodine intake in the increase of goiter and thyroid volume. Significant increase in thyroid volume and goiter prevalence were found in patients with UIC > 300 μg/L [23, 24].
In a study conducted in Switzerland, there was no increase at thyroid volume in the UIC 300–500 μg/L range; UIC > 500 μg/L was associated with increased thyroid volume [25]. Barrere et al. found a different result, urinary iodine excretion of thyroid volume was found to have negative correlation [26]. In a study conducted in Kayseri in our country, 65 % goiter was detected in adults with palpation; UIC significantly lower in patients with goitre [27].
When the studies on the relationship between age and UIC were examined in the literature, different results were found. In a population-based study of various age groups, UIC was found to be significantly lower in adolescent women and elderly patients. It’s thought that the decrease in adolescent age group may be related to puberty or gestation secondary to pregnancy and in the elderly group may be caused by the limited salt intake [28].
In our study, as the mean age increased, prevalence of HT increased in accordance with the literature. Lack of relationship between age and UIC may be caused by differences in nutritional status and decreasing iodine salt as age progresses.
TSH, fT3 and fT4 are not considered sensitive indicators of the population iodine status [29]. Several studies reported the relationship between UIC and thyroid functions. Many studies reported that the serum TSH levels showed statistically significant as UIC, and iodine restriction was found to be appropriate to prevent the onset of overt hypothyroidism in the subclinical hypothyroidism [30, 31]. In our study, a significant increase was found in TSH as the UIC increased (p < 0.05). This may be interpreted as iodinated compounds and iodine may have a direct effect on TSH. The increase in population-based studies in our country will help to explain the effects of iodine levels on thyroid functions more reliably.
Conclusions
In conclusion, no relation was found between HT and urine iodine levels in our study. However, there were some limitations such as including the small numbers of patients and the urinary iodine levels of the patients were measured from a single urine sample. Therefore, we think that it would be more appropriate to plan the study with repeated measures in the wider patient group. Despite all these limitations, we think that it is important to know the characteristics of the region in approach to HT patients. HT is quite common even in the regions where iodine deficiency is still present, such as our country. When investigating the etiology of the disease, it should be remembered that may be there are more important genetic and environmental factors than iodine intake.
For this reason, it should be considered that iodine deficiency may be more harmful to the patient before the iodine restriction is recommended to protect against HT by looking at other studies in the literature.
Conflicts of interests. Authors declare the absence of any conflicts of interests and their own financial interest that might be construed to to influence the results or interpretation of their manuscript.
Ethical approval. Consent forms and protocols were approved by the Ethical Committee of Adana Numune Education and Research Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent. Informed consent was obtained from all individual participants included in the study.
Data availability statement. The computer registered data used to support the findings of this study are available from the corresponding author upon request.
Funfing statement. This study was funded by Ege University Scientific Research Projects Coordinator.
HT; Hashimoto’s thyroiditis, fT3; serum free-triiodothyronine, fT4; serum free-thyroxine, TSH; thyroid stimulating hormone, Anti-TG; anti-thyroglobulin antibody, Anti-TPO; anti-thyroid peroxidase antibody, UIC; urinary iodine concentration, μg/L; microgram/liter
Список литературы
1. Hollowell J.G., Staehling N.W., Flanders W.D., Hannon W.H., Gunter E.W. et al. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 2002. 87. 489-499.
2. Amino N., Tada H., Hidaka Y., Hashimoto K. Hashimoto’s disease and Dr. Hakaru Hashimoto. Endocr. J. 2002. 49. 393-397.
3. Saravanan P., Dayan C.M. Thyroid autoantibodies. Endocrinol. Metab. Clin. North Am. 2001. 30. 315-337, viii.
4. Dayan C.M., Daniels G.H. Chronic autoimmune thyroiditis. N. Engl. J. Med. 1996. 335. 99-107.
5. Chistiakov D.A. Immunogenetics of Hashimoto’s thyroiditis. J. Autoimmune Dis. 2005. 2. 1.
6. Andersen S., Karmisholt J., Pedersen K.M., Laurberg P. Reliability of studies of iodine intake and recommendations for number of samples in groups and in individuals. Br. J. Nutr. 2008. 99. 813-818.
7. World Health Organization, UNICEF & International Council for Control of Iodine Deficiency Disorders. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers, 3rd ed. Geneva: WHO, 2007.
8. Knudsen N., Christiansen E., Brandt-Christensen M., Nygaard B., Perrild H. Age- and sex-adjusted iodine/creatinine ratio. A new standard in epidemiological surveys? Evaluation of three different estimates of iodine excretion based on casual urine samples and comparison to 24 h values. Eur. J. Clin. Nutr. 2000. 54. 361-363.
9. Ebner S.A., Lueprasitsakul W., Alex S., Fang S.L., Appel M.C., et al. Iodine Content of Rat Thyroglobulin Affects its Antigenicity in Inducing Lymphocytic Thyroiditis in the BB/Wor Rat AU. Autoimmunity. 1992. 13. 209-214.
10. Pino S., Fang S.L., Braverman L.E. Ammonium persulfate: a safe alternative oxidizing reagent for measuring urinary iodine. Clin. Chem. 1996. 42. 239-243.
11. Zois C., Stavrou I., Kalogera C., Svarna E., Dimoliatis I. et al. High prevalence of autoimmune thyroiditis in schoolchildren after elimination of iodine deficiency in northwestern Greece. Thyroid. 2003. 13. 485-489.
12. Zhang J.Y., Li S.M., Leng J.L., Chen Y.J., Pu J. et al. Changes of the spectrum on thyroid disease after the ten-year implementation of universal salt iodization in Guangxi Zhuang Autonomous Region. Zhonghua Liu Xing Bing Xue Za Zhi. 2013. 34. 970-974.
13. Camargo R.Y., Tomimori E.K., Neves S.C., GS Rubio I., Galrão A.L. et al. Thyroid and the environment: exposure to excessive nutritional iodine increases the prevalence of thyroid disorders in Sao Paulo, Brazil. Eur. J. Endocrinol. 2008.159. 293-299.
14. Palaniappan S., Shanmughavelu L., Prasad H.K., Subramaniam S., Krishnamoorthy N. et al. Improving iodine nutritional status and increasing prevalence of autoimmune thyroiditis in children. Indian J. Endocrinol. Metab. 2017. 21. 85-89.
15. Pedersen I.B., Knudsen N., Carlé A., Vejbjerg P., Jørgensen T. et al. A cautious iodization programme bringing iodine intake to a low recommended level is associated with an increase in the prevalence of thyroid autoantibodies in the population. Clin. Endocrinol. (Oxf.). 2011. 75. 120-126.
16. Laurberg P., Bülow Pedersen I., Knudsen N., Ovesen L., Andersen S. Environmental iodine intake affects the type of nonmalignant thyroid disease. Thyroid. 2001. 11. 457-469.
17. Xu C., Wu F., Mao C., Wang X., Zheng T. et al. Excess iodine promotes apoptosis of thyroid follicular epithelial cells by inducing autophagy suppression and is associated with Hashimoto thyroiditis disease. J. Autoimmun. 2016. 75. 50-57.
18. Zimmermann M.B., Moretti D., Chaouki N., Torresani T. Introduction of iodized salt to severely iodine-deficient children does not provoke thyroid autoimmunity: a one-year prospective trial in northern Morocco. Thyroid. 2003. 13. 199-203.
19. Erdoğan M.F., Ağbaht K., Altunsu T., Ozbaş S., Yücesan F. et al. Current iodine status in Turkey. J. Endocrinol. Invest. 2009. 32. 617-622.
20. Darcan S., Unak P., Yalman O., Lambrecht F.Y., Biber F.Z. et al. Determination of iodine concentration in urine by isotope dilution analysis and thyroid volume of school children in the west coast of Turkey after mandatory salt iodization. Clin. Endocrinol. (Oxf.). 2005. 63. 543-548.
21. Demircan H.Ç., Kadioglu U.O. Autoimmune thyroiditis diet with case urinary iodine levels of salt iodization in the relationship between levels of zz İzmir E.A.H Tip. Journal. 2005. 19. 6-14.
22. Erdogan M., Erdem N., Cetinkalp S., Ozgen A.G., Saygılı F. et al. Demographic, clinical, laboratory, ultrasonographic, and cytological features of patients with Hashimoto’s thyroiditis: results of a university hospital of 769 patients in Turkey. Endocrine. 2009. 36. 486-490.
23. Chen W., Li X., Wu Y., Bian J., Shen J. et al. Associations between iodine intake, thyroid volume, and goiter rate in school-aged Chinese children from areas with high iodine drinking water concentrations. Am. J. Clin. Nutr. 2017. 105. 228-233.
24. Henjum S., Barikmo I., Gjerlaug A.K., Mohamed-Lehabib A., Oshaug A. et al. Endemic goitre and excessive iodine in urine and drinking water among Saharawi refugee children. Public Health Nutr. 2010. 13. 1472-1477.
25. Zimmermann M.B., Ito Y., Hess S.Y., Fujieda K., Molinari L. High thyroid volume in children with excess dietary iodine intakes. Am. J. Clin. Nutr. 2005. 81. 840-844.
26. Barrère X., Valeix P., Preziosi P., Bensimon M., Pelletier B. et al. Determinants of thyroid volume in healthy French adults participating in the SU.VI.MAX cohort. Clin. Endocrinol. (Oxf). 2000. 52. 273-278.
27. Bayram F., Beyazyildiz A., Gökçe C., Budak N., Erdoğan N. et al. The prevalence of iodine deficiency, serum thyroglobulin, anti-thyroglobulin and thyroid peroxidase antibody levels in the urban areas of Kayseri, Central Anatolia. Exp. Clin. Endocrinol. Diabetes. 2009. 117. 64-68.
28. Als C., Keller A., Minder C., Haldimann M., Gerber H. Age- and gender-dependent urinary iodine concentrations in an area-covering population sample from the Bernese region in Switzerland. European Journal Endocrinology. 2000. 143. 629-637.
29. Pearce E.N., Caldwell K.L. Urinary iodine, thyroid function, and thyroglobulin as biomarkers of iodine status. Am. J. Clin. Nutr. 2016. 104. 898-901.
30. Kim H.I., Oh H.K., Park S.Y., Jang H.W., Shin M.H., Kim S.W., Kim T.H., Chung J.H. Urinary iodine concentration and thyroid hormones: Korea National Health and Nutrition Examination Survey 2013–2015. Eur. J. Nutr. 2019. 58 (1). 233-240. doi: 10.1007/s00394-017-1587-8.
31. Joung J.Y., Cho Y.Y., Park S.M., Kim T.H., Kim N.K., Sohn S.Y., Kim S.W., Chung J.H. Effect of iodine restriction on thyroid function in subclinical hypothyroid patients in an iodine-replete area: a long period observation in a large-scale cohort. Thyroid. 2014. 24 (9). 1361-8.
/11_2.jpg)

/11.jpg)