Журнал "Гастроэнтерология" Том 53, №3, 2019
Вернуться к номеру
Характеристика стану кишкової мікрофлори та вмісту коротколанцюгових жирних кислот у хворих із синдромом подразненого кишечника
Авторы: Yu.M. Stepanov(1), I.Ya. Budzak(2), I.A. Klenina(1)
(1) — State Institution “Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine”, Dnipro, Ukraine
(2) — State Institution “Dnipropetrovsk Medical Academy of the Ministry of Health of Ukraine”, Dnipro, Ukraine
Рубрики: Гастроэнтерология
Разделы: Клинические исследования
Версия для печати
У статті розглядаються питання, пов’язані з кишковою мікрофлорою та коротколанцюговими жирними кислотами у хворих із синдромом подразненого кишечника. Встановлено, що концентрація оцтової, пропіонової, масляної кислот у калі є вищою у хворих із синдромом подразненого кишечника з діареєю порівняно з хворими без діарейного синдрому. У 83,3–88,9 % хворих із різними формами синдрому подразненого кишечника спостерігаються дисбіотичні зміни; у пацієнтів із діарейною формою частіше відзначається знижений вміст біфідобактерій та, особливо, лактобактерій. Виявлена певна взаємозалежність між концентрацією коротколанцюгових жирних кислот та вмістом біфідобактерій, лактобактерій, кандидозної та умовно-патогенної флори.
В статье рассматриваются вопросы, связанные с кишечной микрофлорой и короткоцепочечными жирными кислотами у больных с синдромом раздраженного кишечника. Установлено, что концентрация уксусной, пропионовой, масляной кислот в кале выше у больных с синдромом раздраженного кишечника с диареей по сравнению с больными без диарейного синдрома. У 83,3–88,9 % больных с различными формами синдрома раздраженного кишечника наблюдаются дисбиотические изменения; у пациентов с диарейной формой чаще отмечается пониженное содержание бифидобактерий и, особенно, лактобактерий. Отмечается определенная взаимозависимость между концентрацией короткоцепочечных жирных кислот и содержанием бифидобактерий, лактобактерий, кандидозной и условно-патогенной флоры.
The article presents the issues related to gut microbiota and short-chain fatty acids in patients with irritable bowel syndrome. It was found that the faecal concentration of acetic, propionic, butyric acids is higher in patients with irritable bowel syndrome with diarrhea, compared with the patients without diarrhea syndrome. 83.3–88.9% of patients with various forms of irritable bowel syndrome presented with dysbiotic changes. The reduced concentration of Bifidobacterium species, mainly lactic acid bacteria, is more often registered in patients with diarrheal form. There is a certain correlation between the concentration of short-chain fatty acids and the content of Bifidobacterium, Lactobacillus, Candida and opportunistic bacteria.
синдром подразненого кишечника; мікрофлора кишечника; дисбіоз; коротколанцюгові жирні кислоти
синдром раздраженного кишечника; микрофлора кишечника; дисбиоз; короткоцепочечные жирные кислоты
irritable bowel syndrome; gut microbiota; dysbiosis; short-chain fatty acids
Introduction
Many studies have found that impaired gut microbiota is an important component of the development of irritable bowel syndrome (IBS). Throughout the gut, microbiota plays an important role in the normal functioning of the gut. Molecular technologies have established the dominance of four classes of microorganisms: Firmicutes (64 %), Bacteroidetes (23 %), Proteobacteria (8 %), Actinobacteria (3 %) [1].
In recent decades, a large number of studies have been conducted to investigate the incidence of dysbiosis in patients with IBS, as well as the nature of changes in the individual composition of the gut microbiota. In a famous study by Casén C. et al. (2015), which was conducted in Sweden, Norway, Denmark and Spain, intestinal dysbiosis was detected by genetic methods in 73 % of IBS patients and 16 % of healthy individuals [2].
The studies examining the species composition of microbiota in IBS have shown varied results, given the different techniques used in these studies. However, the general trend is a decrease in Firmicutes and Bacteroidetes with different ratios depending on the form of IBS. Among these classes of bacteria, the content of Lactobacillus and Bifidobacterium is most commonly decreased, especially in the diarrheal form of IBS [1].
In contrast, many studies have shown an increase of other microorganisms in IBS, including Pseudomonas aeruginosa, Ruminococcus spp., Escherichia, Clostridium spp., Streptococcus spp. and others. This category of patients demonstrated the increased levels of C-reactive protein, proinflammatory cytokines (IL-6 and IL-8), bacterial lipopolysaccharides [3].
The question is, how do the altered gut microbiota impact IBS development?
Microbiota performs several important functions. First of all, it concerns the prevention of infectious factors. In the intestine, there is a constant fight or “competition” between the beneficial commensals (symbionts) to which Firmicutes and Bacteroidetes belong, with pathogenic or potentially pathogenic pathobionts. This “competition” is called colonization resistance and is an important guarantee of the normal functioning of the gastrointestinal system. Certain important molecules, which may include short-chain fatty acids (SCFA) and bacteriocins, are implicated in the course of colonization resistance [4].
Researchers’ interest in SCFA has been around for decades. Back in 1980, Roediger W.E. found that SCFA produced by anaerobic bacteria are an important source of energy for colonocytes [5].
Since that time there were performed many studies which revealed important diverse functions of SCFA, including the effect on intestinal motor activity, stimulation of the immune system, blocking the activation of pathogenic flora, impact on metabolic processes. SCFA lowers the pH around the intestinal epithelial cells that has a protective effect on them. Besides, SCFA provide antibacterial protection against pathogenic bacteria by attracting neutrophils and cytokines, with immune tolerance to commensal flora remaining [4]. It is believed that different SCFA can have various effects, in particular, butyrate is more present in the intestine, propionate — in the enterohepatic circulation, acetate — in the systemic circulation [4, 6].
The article reviews the results of some recent studies.
The experimental research by Wang H.-B. et al. studied the anti-inflammatory properties of intestinal bacteria, in particular in acute systemic inflammation due to septic shock. Butyrate (butyric acid) has been found to reduce the plasma levels of proinflammatory compounds TNF-α, IL-6 and IL-1β in the experimental animals; however, butyrate significantly increases the anti-inflammatory IL-10 [7]. The study by Tedelind S. et al. (2007) found anti-inflammatory effects of other SCFA: acetate, propionate, butyrate reduced TNF-α release stimulated by lipopolysaccharide [8].
Butyrate also stabilizes the intestinal barrier function, in particular by influencing hypoxia-inducible factor [9]. In a Chinese study, Feng Y. et al. (2018) found that SCFA stimulate the formation of the intestinal epithelial barrier and protect it against damage by lipopolysaccharides, in particular through inhibition of NLRP3 inflammasome and autophagy [10].
The famous Spanish study of Pozuelo M. et al. (2015) investigated the microbiome and SCFA in 113 patients with IBS and 66 healthy individuals. With the use of 16S rRNA genetic research methods, it was found that IBS was associated with a reduced heterogeneity of microorganisms, as well as a decrease in butyrate-producing bacteria, especially in patients with diarrheal and mixed forms of IBS [11]. A decrease in butyrate production can augment intestinal permeability, enhance nociceptive sensory response, and exacerbate IBS symptoms [1].
Given the multifactorial nature of the mechanisms of IBS development, the diverse action of SCFA may be important in IBS. SCFA is believed to be an important component of maintaining intestinal and immune homeostasis [12]. However, many questions about the SCFA and the development of IBS remain unclear.
Purpose of the study was to investigate the features of SCFA faecal content in IBS patients depending on dysbiotic changes in the gut microbiome.
Materials and methods
The study was conducted at the State Institution “Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine”. The study involved 15 IBS patients. The diagnosis of irritable bowel syndrome was established after a thorough clinical and anamnestic examination, taking into account compliance with the Rome IV criteria (2016) with the exclusion of anxiety symptoms. All patients experienced intestinal pain. Other symptoms include bloating and abdominal distension; some patients presented with anxiety. Attention was first of all paid to the nature of the emptying. According to the Bristol Stool Chart, patients had diarrheal IBS (9 patients) and non-diarrheal forms (with or without constipation) (6 patients).
All patients enrolled in the study were evaluated for SCFA content. The SCFA level was measured by the chromatographic method using the hardware-software complex for medical research based on the gas chromatograph “Chromatec-Crystal 5000” by the method of Guohua Zhao [13]. The quantitative identification of the SCFA fractions (µg/mg) of acetic (C2), propionic (C3), butyric (C4) acids, column calibration, and chromatogram calculation were performed by the method of normalization of peak areas and their fractions according to the standards of “Sigma-Aldrich Acids” (USA).
Besides, all patients underwent bacteriological (cultural) study of feces with the determination of the gut microbiota composition (the content of Bifidobacteria, Lactobacilli, Escherichia, Enterococci, potentially pathogenic and Candida flora). Investigation of the species and quantitative composition of the colonic microbiota was performed using ten-fold dilutions (10–1–10–9) on a standard set of elective and differential diagnostic nutrient media for isolation of aerobic and anaerobic microorganisms.
Results ans discussion
According to the results of the determination of faecal SCFA content, the level of acetic acid (C2) in patients with diarrheal IBS varied within a range of 0–0.461 µg/mg; the average level was (0.236 ± 0.044) µg/mg. The content of acetic acid (C2) in IBS patients with no diarrhea ranged 0.034–0.251 µg/mg; the average value was (0.120 ± 0.041) µg/mg (p = 0.039).
The concentration of propionic acid (C3) in patients with diarrhea ranged from 0.003 to 0.229 µg/mg; the average value was (0.074 ± 0.028) µg/mg. The propionic acid content (C3) in IBS without diarrhea was 0.010–0.114 µg/mg; the average value was (0.041 ± 0.016) µg/mg (p = 0.162).
The content of butyric acid (C4) in diarrheal form ranged 0–0.106 µg/mg; the average value was (0.051 ± 0.012) µg/mg. Instead, the patients with diarrhea-free IBS had an average concentration of butyric acid (C4) of (0.033 ± 0.009) μg/mg, with fluctuations ranging of 0.010–0.060 μg/mg (p = 0.116).
Thus, the concentration of all SCFA in IBS patients is higher in the presence of diarrhea compared with the patients without diarrhea (Fig. 1). It is also noticeable that the acetic acid content is the highest.
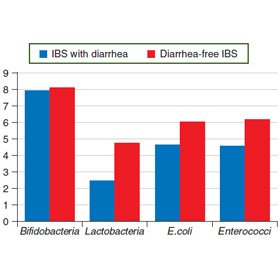
The bacteriological examination found the signs of intestinal dysbiosis in 88.9 % patients with diarrheal IBS and 83.3 % patients with non-diarrheal IBS. The average content of Bifidobacteria (logarithm) did not differ for diarrheal and non-diarrheal forms of IBS and accounted for (8.67 ± 0.24) and (8.67 ± 0.33), respectively. The Lactobacilli content (logarithm) was lower in diarrheal form (4.67 ± 0.37) compared with non-diarrheal (5.33 ± 0.21). The content of E.coli with normal properties between the groups differed slightly: (6.44 ± 0.24) for diarrhea, (6.67 ± 0.67) for non-diarrheal form. The Enterococci content was slightly lower in diarrheal form (6.33 ± 0.29) than in the absence of diarrhea (7.17 ± 0.31) (Fig. 2).
In patients with diarrhea, a decrease in Bifidobacteria content was observed in 22.2 % of cases, and a decrease in Lactobacilli content in 66.7 % people. In 16.7 % patients without diarrhea, the Bifidobacteria content was reduced; there was no reduction in Lactobacilli concentration. In 55.6 % of patients with diarrheal syndrome and 50.0 % of diarrhea-free patients, an increase in the concentration of Candida was observed; 66.7 % people with diarrhea and 100 % diarrhea-free patients presented with an increase in potentially pathogenic flora.
The selection of SCFA was estimated depending on the disturbances of the gut microbiota.
By reducing the release of Bifidobacteria, the faecal content of SCFA was: acetic acid (0.220 ± 0.092) μg/mg, propionic acid (0.084 ± 0.073) μg/mg, butyric acid (0.068 ± ± 0.025) μg/mg. Instead, with normal bifidobacterial content, the concentration of acetic acid was (0.182 ± ± 0.037) μg/mg, propionic acid (0.055 ± 0.015) μg/mg, and butyric acid (0.038 ± 0.007) μg/mg. With the reduced content of Lactobacilli, the selection of SCFA was as follows: C2 — (0.2000 ± 0.0635) µg/mg, C3 — (0.072 ± 0.026) µg/mg, C4 — (0.046 ± 0.011) µg/mg; at the normal content of Lactobacilli: C2 — (0.183 ± 0.041) µg/mg, C3 — (0.054 ± 0.024) µg/mg, C4 — (0.042 ± 0.011) µg/mg. Thus, there is a tendency for an increased selection of SCFA in patients with a low content of Bifidobacteria and Lactobacilli compared to the normal microbiota, but this difference is insignificant.
With the increase of the Candida flora, the SCFA content was slightly higher than in the absence of candidiasis: the content of acetic acid was (0.219 ± 0.054) μg/mg in candidiasis, (0.156 ± 0.041) μg/mg without candidiasis; propionic acid content accounted for (0.091 ± 0.029) μg/mg in candidiasis, (0.028 ± 0.010) μg/mg without candidiasis; butyric acid content was (0.055 ± 0.012) µg/mg in candidiasis, (0.031 ± 0.009) µg/mg without candidiasis. On the contrary, in patients with increased potentially pathogenic flora, the SCFA content was lower than in the absence of increased potentially pathogenic flora: C2 (0.170 ± ± 0.040) μg/mg and (0.267 ± 0.031) μg/mg, respectively; C3 (0.047 ± 0.016) μg/mg and (0.118 ± 0.056) μg/mg, respectively; C4 (0.040 ± 0.008) μg/mg and (0.061 ± ± 0.024) μg/mg, respectively.
Thus, in patients with IBS, there is a certain interaction between the state of gut microbiota and the faecal content of SCFA: the increase in the concentration of SCFA is slightly higher with a decrease in Bifidobacteria and Lactobacilli and increase of the Candida flora; on the contrary, the decrease in the concentration of SCFA may be established in case of increased potentially pathogenic flora. Further study of the relationship between the state of the gut microbiota, the nature of dysbiotic changes, and the release of SCFA is needed.
Conclusions
1. The faecal concentration of SCFA is higher in IBS patients with diarrhea compared with the patients without diarrheal syndrome.
2. 83.3–88.9 % of patients with various forms of IBS presented with gut dysbiosis; the patients with diarrhea are more likely to have a reduced content of Bifidobacteria and, in particular, Lactobacilli.
3. There is a certain relationship between the SCFA concentration and the content of Bifidobacteria, Lactobacilli, Candida and potentially pathogenic flora, which need to be evaluated in further investigations.
Conflicts of interests. Authors declare the absence of any conflicts of interests and their own financial interest that might be construed to influence the results or interpretation of their manuscript.
1. Chong P.P., Chin V.K., Looi C.Y., Wong W.F., Madhavan P., Yong V.C. The Microbiome and Irritable Bowel Syndrome — A Review on the Pathophysiology, Current Research and Future Therapy [published correction appears in Front Microbiol. 2019 Aug 13;10:1870]. Front Microbiol. 2019. 10. 1136.
2. Casén C., Vebø H.C., Sekelja M., Hegge F.T., Karlsson M.K., Ciemniejewska E. et al. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment. Pharmacol. Ther. 2015. 42. 71-83.
3. Iacob S., Iacob D.G. Infectious Threats, the Intestinal Barrier, and Its Trojan Horse: Dysbiosis. Front Microbiol. 2019. 10. 1676.
4. Ludidi S., Jonkers D., Elamin E., Pieters H.J., Schaepkens E., Bours P. et al. The intestinal barrier in irritable bowel syndrome: subtype-specific effects of the systemic compartment in an in vitro model. PLoS One. 2015. 10. e0123498.
5. Roediger W.E. Role of anaerobic bacteria in the metabolic welfare of the colonic mucosa in man. Gut. 1980. 21. 793-798.
6. Fukuda S., Toh H., Hase K., Oshima K., Nakanishi Y., Yoshimura K. et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011. 469. 543-547.
7. Wang H.-B., Wang P.-Y., Wang X., Wan Y.-L., Liu Y.-C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig. Dis. Sci. 2012. 57. 3126-3135.
8. Tedelind S., Westberg F., Kjerrulf M., Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007. 13 (20). 2826-2832.
9. Kelly C.J., Zheng L., Campbell E.L., Saeedi B., Scholz C.C., Bayless A.J. et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host. Microbe. 2015. 17. 662-671.
10. Feng Y., Wang Y., Wang P., Huang Y., Wang F. Short-chain fatty acids manifest stimulative and protective effects on intestinal barrier function through the inhibition of NLRP3 inflammasome and autophagy. Cell. Physiol. Biochem. 2018. 49. 190-205.
11. Pozuelo M., Panda S., Santiago A., Mendez S., Accarino A., Santos J. et al. Reduction of butyrate- and methane-producing microorganisms in patients with irritable bowel syndrome. Sci. Rep. 2015. 5. 12693.
12. Tan J., McKenzie C., Potamitis M., Thorburn A.N., Mackay C.R., Macia L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014. 121. 91-119.
13. Guohua Zhao. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomedical chromatography. 2006. 20. 8. 675-682.

/49-1.jpg)