Журнал "Гастроэнтерология" Том 53, №4, 2019
Вернуться к номеру
Нейровегетативні та когнітивні порушення в пацієнтів iз неалкогольною жировою хворобою печінки i цукровим діабетом 2 типу
Авторы: Ye.S. Sirchak, O.O. Boldizhar, V.I. Griga, O.I. Petrichko
Uzhhorod National University, Uzhhorod, Ukraine
Рубрики: Гастроэнтерология
Разделы: Клинические исследования
Версия для печати
Актуальність. Проводяться різноспрямовані дослідження для кращого розуміння впливу когнітивних порушень на лікування, мобільність, смертність пацієнтів, особливо осіб iз поліморбідною патологією. Мета: вивчити особливості когнітивних i нейровегетативних порушень у пацієнтів iз різними формами неалкогольної жирової хвороби печінки (НАЖХП) (неалкогольний жировий гепатоз (НАЖГ) та неалкогольний стеатогепатит (НАСГ)) та цукровим діабетом 2 типу. Матеріали та методи. Обстежено 50 пацієнтів iз НАЖХП (24 — з НАЖГ та 26 — з НАСГ). Група порівняння включала 22 людини з ЦД 2 типу. Порушення функцій центральної та вегетативної нервової системи визначали за допомогою нейропсихометричних тестів. Результати. В обстежених пацієнтів за результатами анкетування встановлено вегетативну дисфункцію. Когнітивні порушення (переважно легкі та помірні) виявлено в обох групах хворих. Максимально виражені порушення визначались у сфері уваги і концентрації, пам’яті, менш виражені — у зорово-конструктивній сфері, особливо у хворих iз НАСГ у поєднанні з ЦД 2 типу. Стресові ситуації мають виражений вплив на життя цих пацієнтів, при цьому вони не досить критично реагують на виявлені проблеми. У хворих iз НАСГ у поєднанні з ЦД 2 типу встановлено найнижчий рівень опірності стресу — (56.7 ± 3.1) бала; p < 0,01. У них виявлена легка вразливість навіть за незначного негативного впливу. Висновки. У пацієнтів iз НАЖХП та ЦД 2 типу виявлено когнітивні порушення та вегетативну дисфункцію за результатами нейропсихометричного тестування. Найбільш вираженими когнітивні порушення були у хворих iз НАСГ у поєднанні з ЦД 2 типу та ожирінням І ступеня за результатами Монреальської шкали оцінки когнітивних функцій (r = 0,90; р < 0,01), Бостонського тесту (r = 0,94; р < 0,01) та Вісконсинського тесту сортування карток (r = 0,92; р < 0,01).
Актуальность. Проводятся разнонаправленные исследования для лучшего понимания влияния когнитивных нарушений на лечение, мобильность, смертность пациентов, особенно лиц с полиморбидной патологией. Цель: изучить особенности когнитивных и нейровегетативных нарушений у пациентов с различными формами неалкогольной жировой болезни печени (НАЖБП) (неалкогольный жировой гепатоз (НАЖГ) и неалкогольный стеатогепатит (НАСГ)) и сахарным диабетом (СД) 2 типа. Материалы и методы. Обследовано 50 пациентов с НАЖБП (24 — с НАЖГ и 26 — с НАСГ). Группа сравнения включала 22 человека с СД 2 типа. Нарушения функций центральной и вегетативной нервной системы определяли с помощью нейропсихометрических тестов. Результаты. У обследованных пациентов по результатам анкетирования установлена вегетативная дисфункция. Когнитивные нарушения (преимущественно легкие и умеренные) выявлены в обеих группах больных. Максимально выраженные нарушения определялись в сфере внимания и концентрации, памяти, менее выраженные — в зрительно-конструктивной сфере, особенно у больных с НАСГ в сочетании с СД 2 типа. Стрессовые ситуации негативно влияют на жизнь этих пациентов, при этом они недостаточно критически реагируют на обнаруженные проблемы. У больных НАСГ в сочетании с СД 2 типа установлен наименьший уровень сопротивляемости стрессу — (56,7 ± 3,1) балла; p < 0,01. У них определяется легкая уязвимость даже при незначительном негативном влиянии. Выводы. У пациентов с НАЖБП и СД 2 типа выявлены когнитивные нарушения и вегетативная дисфункция по результатам нейропсихометрического тестирования. Наиболее выраженными когнитивные нарушения были у больных НАСГ в сочетании с СД 2 типа и ожирением I степени по результатам Монреальской шкалы оценки когнитивных функций (r = 0,90; р < 0,01), Бостонского теста (r = 0,94; р < 0,01) и Висконсинского теста сортировки карточек (r = 0,92, р < 0,01).
Background. There are multifunctional researches for better understanding the effect of cognitive disorders on treatment, mobility, mortality of patients, especially persons with multimorbid pathology. The purpose was to study the features of cognitive and neurovegetative dysfunction in patients with different forms of non-alcoholic fatty liver disease (NAFLD) (non-alcoholic fatty hepatosis (NAFH) and non-alcoholic steatohepatitis (NASH)) and type 2 diabetes mellitus (T2DM). Materials and methods. Fifty patients with NAFLD (24 — with NAFH and 26 — with NASH) were examined. The comparison group included 22 patients with T2DM. Dysfunction of central and autonomic nervous system was evaluated with the help of neuropsychometric testing. Results. The analysis of conducted research indicates autonomic dysfunction in the surveyed patients. Cognitive impairment (mostly mild and moderate) was detected in all persons in both groups. The most pronounced disturbances were determined in the areas of attention and concentration, memory, less pronounced — in visually constructive area, especially in patients with NASH combined with type 2 diabetes. Stressful situations have a significant impact on the lives of these persons, but they are not critical enough to any issues. Patients with NASH associated with type 2 diabetes have the lowest level of resistance to stress — (56.7 ± 3.1) points; p < 0.01. They experienced a slight vulnerability even from minor impacts. Conclusions. According to neuropsychometric testing, cognitive impairment and autonomic dysfunction were found in patients with NAFLD and type 2 diabetes. The most pronounced cognitive impairment was found in patients with NASH combined with type 2 diabetes and obesity class I, according to the results of the Montreal Cognitive Assessment (r = 0.90; p < 0.01), the Boston test (r = 0.94; p < 0.01) and the Wisconsin Card Sorting Test (r = 0.92; p < 0.01).
неалкогольна жирова хвороба печінки; цукровий діабет 2 типу; когнітивні порушення; вегетативна дисфункцiя
неалкогольная жировая болезнь печени; сахарный диабет 2 типа; когнитивные нарушения; вегетативная дисфункция
non-alcoholic fatty liver disease; type 2 diabetes mellitus; cognitive impairment; autonomic dysfunction
Introduction
The challenges faced by people living with multiple chronic conditions are unique for patients with dementia and other cognitive impairment [1]. The term “cognitive impairment” is used to describe a deficit beyond that associated with normal aging, but not characterized as dementia. Cognitive impairment, or cognitive disorder, is an impairment of memory and at least one other aspect, or domain, of cognitive function, such as language, orientation, reasoning, attention, or executive functioning, the cognitive skill necessary for planning and sequencing tasks. Mild cognitive impairment differs from dementia syndrome and, unlike dementia, has no impact on individual daily activities [2].
The etiology of cognitive impairment is multifactorial. Oxidative stress, immune inflammatory processes, anemia, hyperhomocysteinemia, and vitamin B12 deficiency may be involved in this decline in neurocognitive performance [2] that also can be detected in patients with non-alcoholic fatty liver disease (NAFLD) [3].
There are multifunctional researches for better understanding the effect of cognitive disorders on treatment, mobility, mortality of patients, especially persons with multimorbid pathology. Some trialists think that non-alcoholic fatty liver disease can be well grounded regarded as cognitive-behavioral dysfunction [5].
NAFLD is the leading cause of liver diseases in Western countries, with an overall prevalence of 25 % in the general population, increasing to 70 % in obese people and those who have type 2 diabetes mellitus (T2DM). Moreover, the number of affected individuals is expected to increase in the forthcoming years, in line with increasing obesity due to the adoption of a high-fat diet and sedentary lifestyle [6].
NAFLD is one of the most common chronic liver diseases worldwide [5]. It is characterized by excessive hepatic fat accumulation, associated with insulin resistance, and is defined by the presence of steatosis in > 5 % of hepatocytes according to histological analysis. NAFLD includes two pathologically distinct conditions with different prognoses: non-alcoholic fatty hepatosis (NAFH) and non-alcoholic steatohepatitis (NASH); the latter covers a wide range of disease severity, including fibrosis, cirrhosis and hepatocellular carcinoma. The diagnosis of NAFLD requires exclusion of both secondary causes and a daily alcohol consumption ≥ 30 g for men and 20 g for women [7].
The prevalence and severity of NAFLD is also influenced by the presence of metabolic risk factors, such as overweight/obesity and T2DM [8]. The prevalence of NAFLD and NASH in T2DM is 76 and 22 %, respectively. Furthermore, the prevalence of NAFLD correlates with the degree of impaired glucose metabolism, increasing from 27 % in subjects with normal fasting blood sugar level (fasting blood glucose (FBG) < 6.1 mmol/L) to 43 and 62 %, in those with impaired glucose tolerance (FBG ≥ 6.1 mmol, but < 7 mmol/L) and T2DM (FBG > 7.0 mmol/L) respectively [9].
There are researches studying the psychological status in patients with NAFLD, but they are prevalently directed to detect a depression in these patients, and the obtained results are definitive. In conclusion, the psychological status of patients with NAFLD stays still ambiguous [10], especially in type 2 diabetes mellitus, obesity, etc.
The purpose was to study the features of cognitive and neurovegetative dysfunction in patients with different forms of non-alcoholic fatty liver disease (non-alcoholic fatty hepatosis and non-alcoholic steatohepatitis) and type 2 diabetes mellitus.
Materials and methods
At the clinical site of the Department of Propaedeutics of Internal Diseases of the Medical Faculty of Uzhhorod National University (Gastroenterology and Endocrinology Department of A. Novak Transcarpathian Regional Clinical Hospital), 50 patients with NAFLD and T2DM were examined in 2016–2019. Their average age was (46.3 ± 5.7) years. These patients formed the basic group (group 1). Group 1 was divided into:
— subgroup 1.1 — 24 persons with NAFH: 14 males (58.3 %) and 10 females (41.7 %), the average age was (44.2 ± 7.7) years;
— subgroup 1.2 — 26 individuals with NASH: 15 males (57.7 %) and 11 females (42.3 %), the average age was (48.1 ± 5.3) years.
The comparison group (group 2) included 22 patients with T2DM (without laboratory-instrumental manifestations of liver damage): 13 males (59.1 %) and 9 females (40.9 %), the average age was (43.7 ± 5.2) years.
The control group consisted of 20 apparently healthy persons: 12 males (60.0 %) and 8 females (40.0 %), the average age was (47.6 ± 5.8) years.
All studies were performed with patients’ consent (written consent for performing appropriate diagnostic and therapeutic measures was received from all patients), and the methodology of their implementation was in line with the Helsinki Declaration of Human Rights 1975 and its 1983 revised version, and with the European Convention on Human Rights, as well as biomedicine and legislation of Ukraine.
All the examined patients underwent general clinical, anthropometric, instrumental, and laboratory tests. To verify the diagnosis, the nature of complaints was evaluated, as well as history of disease. In anthropometric study, height, weight, waist circumference were determined, and body mass index (BMI) was calculated. According to the World Health Organization recommendations, patients were divided based on the BMI, where BMI of 16.0 kg/m2 and less corresponded to severe underweight; 16.0–18.5 kg/m2 — underweight; 18.0–24.9 kg/m2 — normal weight; 25.0–29.9 kg/m2 — overweight; 30.0–34.9 kg/m2 — class I obesity; 35.0–39.9 kg/m2 — class II obesity; 40.0 kg/m2 and above — class III obesity (morbid obesity) [11].
Abdominal ultrasound was performed in all patients according to generally accepted method. Standard and biochemical blood tests have been performed to determine the functional state of the liver, lipid and carbohydrate metabolism (glucose, insulin, glycosylated hemoglobin (HbA1c, %)).
NAFLD diagnosis was established in accordance with the unified clinical protocols (Order of the Ministry of Health of Ukraine dated November 6, 2014, no. 826) and European Association for the Study of the Liver, European Association for the Study of Diabetes and European Association for the Study of Obesity Clinical Practice Guidelines for the management of NAFLD. Herewith, the stage of the liver damage was determined on the basis of surrogate laboratory markers of liver fibrosis [12].
Diagnosis of type 2 diabetes mellitus has been established according to the International Diabetes Federation recommendations (2005), as well the taking into account unified clinical protocol (Order of Ministry of Health of Ukraine dated December 21, 2012, no. 1118) [12]. Severity of T2DM was evaluated according to the HbA1c level (norm being up to 6.0 %).
Disorders of the central nervous system in examined patients were determined using the following tests:
1. The Montreal Cognitive Assessment (MоCA) is used for rapid screening for cognitive impairment. It evaluates various cognitive functions: attention and concentration, executive functions, memory, language, visuospatial activity, conceptual thinking, counting and orientation. The survey takes approximately 10 minutes. The maximum score on this scale is 30 points.
2. The Mini-Mental State Examination (MMSE) is a short mental status assessment scale used worldwide to assess cognitive function. It is quite reliable method for primary screening for cognitive impairment, including dementia. The test result is obtained by summing the scores for each item. The maximum figure in this test is 30 points, which corresponds to the highest cognitive ability. The lower the test result, the more marked is cognitive deficit.
3. The technique of “Remembering 10 words” by A. Luria (the study of auditory memory) is used to evaluate the status of auditory memory for words, fatigue, attention activity, memorization, preservation, reproduction, voluntary attention. This technique evaluates auditory short-term and long-term memory. The number of normal memorized units is (7 ± 2) words.
4. The Boston Stress Test, developed by scientists at Boston University Medical Center. It is necessary to answer the questions based on how often these statements are true for the subject of study. The normal reaction to stressful situations indicates the total score of 10 points or less.
5. The Benton Judgment of Line Orientation test (Benton, Hamsher, Varney, and Spreen, 1983) assesses spatial perception. The test includes 5 inclined segments. The patient should find a line with the same slope as in the control map. The Benton test is commonly used in the pathopsychological diagnosis of suspected organic brain damage, as well as for clarifying degree of its severity.
6. The Schulte test is used to examine the distraction, switch, distribution, and focus of the surveyed. It allows you to determine the efficiency (exhaustion), presence or absence of attention disorders. The time of the test is taken into account (the average norm is 40–42 s).
7. The Clock Drawing Test is used to assess cognitive impairment and its severity, as well as possible neurological and psychiatric disorders. The result is rated on a 10-point scale. The lower the score, the more pronounced is the cognitive deficit.
8. The Wisconsin Card Sorting Test (WCST) developed by E. Berg and D. Grant in 1948 allows you to evaluate such cognitive function as the ability: 1) to form abstract categories; 2) to switch attention when changing categories (shifting set); 3) to concentrate attention on the selected category (maintaining set); 4) to use feedback (feedback utilization). The evaluation of results is the absolute and relative number of recognized categories, trials, errors and perseverative errors [13].
Neuropsychometric testing was carried out at intervals of 2–3 hours to restore attention and concentration ability in patients.
Determination of autonomic dysfunction (AD) was carried out with the help of O.M. Wayne questionnaire (1998). A score greater than 15 indicated autonomic dysfunction.
In surveyed patients, the main indicators of hemodynamics (heart rate, blood pressure) were also determined and, based on the obtained data, the Kérdö autonomic index (KAI) was calculated:
KAI = (1 – diastolic blood pressure / heart rate) × 100.
At full autonomic equilibrium (eutonia), the index is in the range –10 to +10. If sympathetic effects (sympathicotonia) are predominant, KAI will be higher than +10, and if parasympathetic effects (vagotonia) are predominant, KAI will be below –10 [14].
For the differential diagnosis of the atherosclerotic changes in the brain vessels in the examined patients, the extracranial portion of the common carotid artery posterior wall intima-media thickness was measured, since this is the marker of carotid atherosclerosis. The normal intima-media thickness for all patients is less than 1.0 mm. If intima-media thickness of the common carotid artery posterior wall exceeded 1.0 mm, the patients were excluded from the study, and the results were considered as manifestations of atherosclerotic lesions of the brain vessels.
Exclusion criteria also were: type 1 diabetes, chronic hepatitis of alcoholic, viral (hepatitis B, C, D) origin, autoimmune hepatitis.
The scientific research was carried out within the scientific research work framework no. 851 “Mechanisms of the formation of complications in liver diseases and pancreas, methods of their treatment and prevention” (state registration number 0115U001103), as well as the general department topic of the Department of Propaedeutics of Internal Diseases.
The analysis and processing of the patient examination results were carried out with the help of computer program Statistica for Windows v.10.0 (StatSoft Inc., USA) using parametric and nonparametric methods. By calculating statistic values, the arithmetic mean (M) and the average error (M ± m) were calculated. The probability of differences between average values was evaluated using the Student’s t-test (normal distribution of value) or with help of Mann-Whitney U test and the Pearson correlation coefficient (for variables).
Results
Patients of groups 1 and 2 had type 2 diabetes of mainly mild severity (compensated carbohydrate metabolism), which was characterized by the presence of relative well-being, lack of hypoglycemic reactions, fasting blood sugar level up to 8.5 mmol/l; blood glucose after a meal up to 10 mmol/l, HbA1c up to 6.5 %.
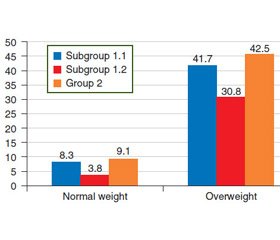
The anthropometric study found that the majority of patients in both groups had overweight or obesity of varying degree (class I–II) (Fig. 1).
It is worth noting that among the patients with NAFH + T2DM (subgroup 1.1), people with overweight prevailed (p < 0.05), while in subgroup 1.2 (NASH + T2DM), class I obesity was detected significantly more often. Class ІІ obesity was also more commonly diagnosed in patients with NASH combined with type 2 diabetes (subgroup 1.2) — 23.1 %.
The analysis of the conducted research indicates the presence of AD in patients of groups 1 and 2 according to the results of a survey (questionnaire by O.M. Wayne), as well as KAI. The main indicators of the functional state of the autonomous nervous system (ANS) are shown in Table 1.
According to the results, the vast majority of patients in both groups complained of a frequent feeling of emotional stress, mood lability, irritability, anxiety, sleep disturbance, headache, fatigue, palpitations, heart failure caused by negative emotions. At the same time, differences were revealed in the degree of AD: the most severe AD, according to the results of Wayne questionnaire, was diagnosed in patients with NASH and type 2 diabetes — (54.5 ± 4.3) points compared to (10.3 ± 1.1) points in the control group, p < 0.01, while patients from the second group had the lowest rate — (31.6 ± 2.9) points.
Further analysis of the functional status of the ANS indicates the predominant influence of its sympathetic component in both groups of examined patients. It should be noted that no statistically significant difference was observed between groups according to KAI.
Neuropsychometric testing made it possible to determine cognitive impairment and to evaluate its severity in patients with NAFLD and type 2 diabetes (Table 2).
Cognitive impairment (mostly mild and moderate) by MoCA was detected in all patients in both groups. The most pronounced disturbances were determined in the areas of attention and concentration, memory, less pronounced — of visual construction, especially in patients of subgroup 1.2.
Analysis of the MMSE results demonstrated that patients of group I had cognitive impairment in the form of mild dementia, with a maximal deviation from the norm in patients with NASH and type 2 diabetes — (21.3 ± 0.5) points; p < 0.05.
Also, the attenuation of attention, increased fatigue, forgetfulness were detected based on the results of A. Luria test in all examined patients, with a maximum deviation from the norm in subgroup 1.2.
Reduced levels of stress persisted according to the results of the Boston test in patients of subgroup 1.1 and group II (p < 0.01). Stressful situations have a pronounced impact on the lives of these patients, but they are not critical enough to any issues. Patients with NASH combined with type 2 diabetes have the lowest level of resistance to stress — (56.7 ± 3.1) points; p < 0.01. They experienced a slight vulnerability even from minor impacts.
Significant memory impairment and inability to focus on specific tasks for a long time, reduced performance, rapid fatigue were diagnosed using Benton, Schulte and Clock Drawing tests. At the same time, only in patients of subgroup 1.2, the received data were statistically significantly different from those of the control group (p < 0.05) according to the results of Benton and Schulte tests.
The inability to switch attention effectively when the environmental conditions are changing was revealed by the WCST in the form of an increase in perseverative responses, which may adversely affect verbal functions in the examined patients with NASH and type 2 diabetes.
So, patients with NAFLD and type 2 diabetes have ANS dysfunction, as well as cognitive impairment, with maximal abnormalities in patients with NASH.
We also analyzed duration of disease in the examined patients (Table 3).
In the majority of patients in subgroup 1.1 (91.7 %), duration of NAFH and type 2 diabetes was up to 5 years (100.0 %), whereas in persons of subgroup 1.2, the disease (NASH) had a longer course — from 6 to 10 years (69.2 % of patients). In this case, patients from subgroup 1.2 were also diagnosed with type 2 diabetes for more than 5 years. Therefore, the progression of liver damage from the stage of non-alcoholic liver steatosis to non-alcoholic steatohepatitis lasting more than 5 years, as well as concomitant metabolic disorders, such as type 2 diabetes, lead to the formation and progression of cognitive impairment and AD in these patients.
Correlation analysis allows us to confirm the involvement of disorders of body weight, namely class I obesity in patients with NASH and type 2 diabetes, into the progression of cognitive impairment. In patients of subgroup 1.2 with overweight, the dependence between BMI disorders and cognitive changes was revealed only while performing the MoCA, A. Luria technique, Boston Stress Test, as well as Clock Drawing Test. At the same time, all correlations were average (Table 4). In subgroup 1.1, a strong correlation was found only in patients with overweight while performing the Boston test. In patients with type 2 diabetes, average correlation was found between overweight and impaired cognitive function by the results of Luria’s and Boston test.
Thus, the results of our studies indicate a significant cognitive impairment and AD in persons with combined pathology, namely NASH, type 2 diabetes and obesity of varying severity, which progress proportionately to the duration of disease in these patients.
Discussion
The results of our studies show a significant cognitive impairment in patients with combined pathology, namely non-alcoholic steatohepatitis, type 2 diabetes mellitus, different stage of obesity, that progresses proportionately to the duration of disease. The use of neuropsychometric tests for detecting cognitive, psycho- and neurovegetative dysfunctions in patients with NAFLD and type 2 diabetes mellitus, according to the results of our study, is an effective method for the diagnosis of nervous system disorder.
Both our data and results of B. Filipovіć et al. (2018) revealed cognitive decline in patients with NAFLD when using MoCA. Studies of K.E. Stewart et al. (2015) found cognitive dysfunction (e.g., memory impairment, attention deficit, etc.) to be significantly more frequent in the overweight/obese NAFLD patients than in the general population [15].
Italian scientists (R. Moretti et al., 2019) also detected disturbances of attention-focusing ability and command execution in patients with NAFLD in the presence of depression and anxiety. In addition to this, those changes were accompanied by a decrease in vitamin B12 level and elevation of homocystein level in serum [3].
The study of S.W. Seo et al. (2016), which included 4,472 participants aged 20–59 years, showed that NAFLD more often occurred in older patients with higher BMI and waist circumference, who had diabetes mellitus, hypertension, hypercholesterolemia, or had acute myocardial infarction or apoplexy. In this category of patients with combined pathology on the background of NAFLD, psychoemotional changes are detected more often according to the results of neuropsychometric tests, which were performed to assess psychomotor speed, visual attention, study function, cognition and concentration. Herewith, an increased activity of alanine aminotransferase and aspartate aminotransferase correlates with more expressed changes in neuropsychometric tests. Our data also show more significant changes of cognitive dysfunctions in patients with non-alcoholic steatohepatitis (than in those with non-alcoholic fatty hepatosis) associated with type 2 diabetes mellitus and obesity.
Attention should be also paid to the lack of consensus on the exact relationship between NAFLD and cognitive dysfunction. There is also being discussed the influence of insulin resistance, oxidative stress, excessive release of inflammatory cytokines and adipokines, endothelial dysfunction and vascular component on formation of neurovegetative and cognitive dysfunction in NAFLD [2, 3, 16].
As our previous researches revealed, patients with NAFLD and diabetes mellitus type 2 had changes in blood flow velocity in the extracranial vessels [17]. Perhaps, disturbance of cerebral blood flow associated with metabolic disease can play the main role in formation of psychoemotional dysfunction in NAFLD. Follow-up studies are needed for more precise understanding of neurovegetative disorders, especially in patients with concomitant diseases (NAFLD, type 2 diabetes mellitus, obesity, etc.), to develop the effective methods for their correction and prevention of progression.
Conclusions
1. In patients with NAFLD and type 2 diabetes, cognitive impairment and autonomic dysfunction were found according to neuropsychometric testing.
2. The most significant cognitive impairment was found in patients with NASH combined with type 2 diabetes and obesity class I, based on the results of MoCA (r = 0.90; p < 0.01), Boston test (r = 0.94; p < 0.01) and WCST (r = 0.92; p < 0.01).
Conflicts of interests. Authors declare the absence of any conflicts of interests and their own financial interest that might be construed to influence the results or interpretation of their manuscript.
Information on contribution of each author: Ye.S. Sirchak — concept and design of the study, analysis of the findings; O.O. Boldizhar — selection of patients; V.I. Griga — collection and processing of the materials, writing the text; O.I. Petrichko — survey of patients.
1. Snowden М.B., Steinman L.E., Bryant L.L. et al. Dementia and co-occurring chronic conditions: a systematic literature review to identify what is known and where are the gaps in the evidence. Int. J. Geriatr. Psychiatry. 2017. № 32(4). Р. 357-371.
2. Gesualdo G.D., Duarte J.G., Zazzetta M.S. et al. Cognitive impairment of patients with chronic renal disease on hemodialysis and its relationship with sociodemographic and clinical characteristics. Dement. Neuropsychol. 2017. № 11(3). Р. 221-226.
3. Moretti R., Caruso P., Gazzin S. Non-alcoholic fatty liver disease and neurological defects. Annals of Hepatology. 2019. № 18. Р. 563-570.
4. Güney Z.Е.O., Sattel H., Witthöft M., Henningsen P. Emotion regulation in patients with somatic symptom and related disorders: A systematic review. PLoS ONE. 2019. № 14(6). Р.1-29. e0217277.
5. Macavei B., Baban A., Dumutrascu D.L. Psychological factors associated with NAFLD/NASH: a systematic review. European Review for Medical and Pharmacological Sciences. 2016. № 20. Р. 5081-5097.
6. Townsend S.A., Newsome P.N. Non-alcoholic fatty liver disease in 2016. British Medical Bulletin. 2016. № 119(1). Р. 143-156.
7. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Obesity facts. European Journal of Obesity. 2016. № 9. Р. 65-90.
8. Степанов Ю.М., Недзвецька Н.В., Ягмур В.Б., Кленіна І.А. Неалкогольна жирова хвороба печінки: особливості метаболічних змін на різних етапах розвитку хвороби. Гастроентерологія. 2018. Т. 52. № 1. С. 13-18.
9. Gan L., Chitturi S., Farrell G.C. Mechanisms and implications of age-related changes in the liver: nonalcoholic fatty liver disease in the elderly. Current Gerontology and Geriatrics Research. 2011. Article ID 831536. 12 p.
10. Filipović B., Marković O., Durić V., Filipović B. Cognitive changes and brain volume reduction in patients with nonalcoholic fatty liver disease. Canadian Journal of Gastroenterology and Hepatology. 2018. Article ID 9638797.
11. WHO: Global Database on Body Mass Index. Available from: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
12. European Association for the Study of the Liver (EASL), European Association for the Study of Diabetes (EASD) and European Association for the Study of Obesity (EASO) EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Journal of Hepatology. 2016. 64. 1388-1402. doi: 10.1016/j.jhep.2015.11.004.
13. Ахутина Т.В., Меликян З.А. Нейропсихологическое тестирование: обзор современных тенденций. К 110-летию со дня рождения А.Р. Лурия. Клиническая и специальная психология. 2012. № 2. С. 1-20. www.psyjournals.ru/psyclin.
14. Насонова Т.І., Кліменко О.В., Колосова Т.В. та ін. Нейровегетативні та когнітивні порушення, асоційовані з тривогою у пацієнтів середнього віку із цереброваскулярними захворюваннями. Семейная медицина. 2017. № 2(70). С. 97-100.
15. Stewart K.E., Haller D.L., Sargeant C. et al. Readiness for behaviour change in non-alcoholic fatty liver disease: implications for multidisciplinary care models. Liver Int. 2015. № 35. Р. 936-943.
16. Seo S.W., Gottesman R.F., Clark J.M. et al. Nonalcoholic fatty liver disease is associated with cognitive function in adults. American Academy of Neurology. 2016. № 86. P. 1136-1142.
17. Sirchak Ye.S., Griga V.I., Kurchak N.Yu., Kutsenko A.Yu. Changes in the brain vessels in patients with non-alcoholic fatty liver disease and carbohydrate metabolism disorders. Гастроентерологія. 2019. Т. 53. № 2. С. 33-40.

/42-1.jpg)
/43-1.jpg)
/44-1.jpg)