Международный эндокринологический журнал Том 15, №8, 2019
Вернуться к номеру
Ренопротекторний ефект метформіну в щурів із цукровим діабетом 2-го типу реалізується шляхом посилення експресії фактора пігментного епітелію в тканині нирок
Авторы: Jiarui Liu (1), Shuangjie Bi (2), Shandong Ye (1)
1 - Anhui Provincial Hospital Affiliated to Anhui Medical University, Hefei, China
2 - The First Affiliated Hospital of USTC, Hefei, China
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
Актуальність. Діабетична нефропатія стала основною причиною термінальної стадії ниркової недостатності. Фактор пігментного епітелію (ФПЕ) є ендогенним протизапальним фактором in vivo, що може пригнічувати експресію патогенних чинників трансформуючого фактора росту β1 и фактора росту сполучної тканини, а також продукцію білка позаклітинного матриксу в нирці за наявності цукрового діабету. Мета дослідження: вивчити вплив метформіну на експресію ФПЕ у тканині нирок щурів із цукровим діабетом 2-го типу і встановити його можливі основні захисні механізми при ураженні нирок. Матеріали та методи. Десять самців щурів лінії Sprague-Dawley були рандомізовані як нормальна контрольна група. Цукровий діабет 2-го типу індукували дієтою з високим вмістом жиру і внутрішньоперитонеальним введенням 30 мг/кг стрептозотоцину. Загалом 30 щурів із діабетом 2-го типу були розподілені на 3 групи, які отримували метформін 300 мг/(кг • д) (група МЕТ, n = 10), глібенкламід 5 мг/(кг • д) (група ГЛІ, n = 10) або фізіологічний розчин (група ФР, n = 10) шляхом введення протягом восьми тижнів. Аналізували різні біохімічні показники, дані гістопатологічного дослідження нирок і рівні експресії ФПЕ у нирковій тканині. Результати. На восьмому тижні рівні глюкози крові натще (ГН), глікованого гемоглобіну (HbA1c), тригліцеридів, екскреції альбуміну із сечею і ФПЕ, сироваткового креатиніну й азоту сечовини крові в групі МЕТ і групі ГЛІ вірогідно знизилися порівняно з групою ФР, вірогідних відмінностей у показниках глюкози крові натщесерце і глікованого гемоглобіну між групою МЕТ і групою ГЛІ не виявлено. При гістологічному дослідженні виявлено поліпшення гломерулярних патологічних змін, спричинених цукровим діабетом, після лікування МЕТ і ГЛІ порівняно з групою ФР. Крім того, зменшувалася екскреція альбуміну із сечею і ФПЕ, спостерігалася менша вираженість патологічних змін у клубочках і підвищувалася експресія білка і мРНК ФПЕ ниркової тканини в групі MЕТ порівняно з групою ГЛІ. Висновки. Метформін знижує екскрецію альбуміну із сечею в діабетичних щурів, покращує морфологію і зменшує вираженість структурних уражень подоцитів. Це може бути частково пов’язане з його роллю у відновленні експресії ФПЕ та інгібуванні екскреції ФПЕ із сечею.
Актуальность. Диабетическая нефропатия стала основной причиной терминальной стадии почечной недостаточности. Фактор пигментного эпителия (ФПЭ) является эндогенным противовоспалительным фактором in vivo, который может подавлять экспрессию патогенных факторов — трансформирующего фактора роста β1 и фактора роста соединительной ткани, а также продукцию белка внеклеточного матрикса в почках при наличии сахарного диабета, что указывает на антифиброгенную активность. Цель исследования: изучить влияние метформина на экспрессию ФПЭ в ткани почек крыс с сахарным диабетом 2-го типа и установить его возможные основные защитные механизмы при поражении почек. Материалы и методы. Десять самцов крыс линии Sprague-Dawley были рандомизированы в качестве нормальной контрольной группы. Сахарный диабет 2-го типа индуцировали посредством диеты с высоким содержанием жира и внутриперитонеального введения 30 мг/кг стрептозотоцина. Всего 30 крыс с диабетом 2-го типа были разделены на 3 группы, которые получали метформин 300 мг/кг/сут (группа MET, n = 10), глибенкламид 5 мг/кг/сут (группа ГЛИ, n = 10) или физиологический раствор (группа ФР, n = 10) в течение восьми недель. Анализировали различные биохимические показатели, данные гистопатологического исследования почек и уровни экспрессии ФПЭ в почечной ткани. Результаты. На восьмой неделе уровни глюкозы крови натощак, гликированного гемоглобина, триглицеридов, экскреции альбумина и ФПЭ с мочой, сывороточного креатинина и азота мочевины крови в группе MET и группе ГЛИ достоверно снизились по сравнению с группой ФР, достоверных различий в показателях глюкозы крови натощак и гликированного гемоглобина между группой MET и группой ГЛИ не обнаружено. При гистологическом исследовании выявлено улучшение гломерулярных патологических изменений, вызванных сахарным диабетом, после лечения MET и ГЛИ по сравнению с группой ФР. Кроме того, уменьшилась экскреция альбумина и ФПЭ с мочой, наблюдалась меньшая выраженность патологических изменений в клубочках и повысилась экспрессия белка и мРНК ФПЭ почечной ткани в группе MET по сравнению с группой ГЛИ. Выводы. Метформин снижает экскрецию альбумина с мочой у крыс с диабетом, улучшает морфологию и уменьшает выраженность структурных поражений подоцитов. Это может быть частично связано с его ролью в восстановлении экспрессии ФПЭ и ингибировании экскреции ФПЭ с мочой.
Background. Diabetic nephropathy has become the primary cause of end-stage renal disease worldwide. Pigment epithelium-derived factor (PEDF) is an endogenous anti-inflammatory factor in vivo, which can inhibit the expression of pathogenic factors — transforming growth factor β1 and connective tissue growth factor, and suppresses extracellular matrix protein production in diabetic kidney, suggesting an antifibrogenic activity. The aim of this study was to investigate the effect of metformin on renal tissue PEDF expression in type 2 diabetic rats and explore its possible underlying protective mechanisms in renal injury. Materials and methods. Ten clean male Sprague-Dawley rats were randomly selected as the normal control group. Type 2 diabetes model were induced by a high-fat diet and intraperitoneal injection of 30 mg/kg streptozotocin. A total of 30 type 2 diabetic rats were divided into 3 groups, which were treated with metformin 300 mg/kg/day (MET group, n = 10), glibenclamide 5 mg/kg/day (GLY group, n = 10), or saline (DM group, n = 10) by gavage for 8 weeks. Various biochemical parameters, kidney histopathology and renal tissue PEDF expression levels were examined. Results. At the 8th week, fasting blood glucose, glycated hemoglobin, triglyceride levels, urinary albumin and PEDF excretion, serum creatinine and blood urea nitrogen in MET group and GLY group decreased significantly compared to DM group, there were no significant differences in fasting blood glucose and glycated hemoglobin between MET group and GLY group. The histological examinations revealed amelioration of diabetes-induced glomerular pathological changes following treatment with MET and GLY when compared to DM group. In addition, urinary albumin and PEDF excretion were decreased, glomerular pathological changes was lightened and protein and mRNA expression of renal tissue PEDF were increased more in MET group compared with GLY group. Conclusions. Metformin reduced urinary albumin excretion in diabetic rats, and improved podocyte morphology and structural damage. The mechanism may be partly related to its role in restoring PEDF expression and inhibiting urinary excretion of PEDF.
цукровий діабет 2-го типу; діабетична хвороба нирок; метформін; фактор пігментного епітелію; глібенкламід
сахарный диабет 2-го типа; диабетическая болезнь почек; метформин; фактор пигментного эпителия; глибенкламид
type 2 diabetes mellitus; diabetic kidney disease; metformin; pigment epithelium-derived factor; glibenclamide
Introduction
Diabetic nephropathy (DN) has become the primary cause of end-stage renal disease worldwide [1]. Pigment epithelium-derived factor (PEDF) is an endogenous anti-inflammatory factor in vivo [2], which can inhibit the expression of pathogenic factors TGF-β1 and CTGF, and suppresses ECM protein production in diabetic kidney, suggesting an antifibrogenic activity [3].
PEDF can reduce albuminuria through the blockade of VEGF expression in the diabetic kidney [2]. In addition, PEDF has been shown to have anti-angiogenic and antioxidant properties in cell culture and animal models [4, 5].
Recently, some researchers reported that metformin, as the first-line hypoglycemic drugs for the treatment of type 2 diabetes mellitus (DM), can prevent and delay the occurrence and progress of diabetic nephropathy through some mechanisms, which include anti-inflammatory, anti-oxidant, anti-fibrosis, and some other non-hypoglycemic action mechanisms [6–8].
In this study, we aim to observe its reno-protective effect and possible underlying mechanisms of metformin in diabetic rats so as to provide an experimental basic for the use of metformin in the clinical prevention and treatment of diabetic nephropathy.
Materials and methods
Reagents
The following reagents were used: STZ (Sigma, USA), metformin and glyburide (Shanghai Shiguibao Medicine Co., Ltd., China), TG, and LDL-C kits (Beijing BHKT Clinical Reagent Co., Ltd.), urine creatinine (Ucr) and urea nitrogen (BUN) kits (Jiancheng Technology Co., Nanjing, China), urinary albumin (Ualb) kit (Tianjing, Xiehe Medicine Co., Ltd., China), urinary PEDF ELISA test kit (Shanghai Dr. Dean Biotechnology Co., China), TRIzol, primers, and real-time PCR kit (TaKaRa, China),Western blot kits (Shanghai Beyotime Biotechnology Co., Ltd.).
Experimental method
Establishment of the experimental mode
We followed the experimental methods of Ye et al. (2015) [9]. Forty-four male SD rats (180–200 g, SPF grade) aged 8 weeks were purchased from the Experimental Animal Center of Anhui Medical University (Anhui, China). All the animals had free access to water under condition of relative humidity of 40–60 % and temperature of 20–30, and were maintained in a 12/12 h light-dark natural cycle during the experiment period. SD rats were fed a high-fat diet (basal diet +10 % lard compound +2 % cholesterol, 50 % calories from fat) for 4 weeks followed by a single intraperitoneal injection of a low dose of streptozotocin (STZ, 30 mg/kg in 10 mmol/L citrate buffer, pH 4.4) after an overnight fast. Normal control rats (n = 10) received an injection the same volume of citrate buffer alone. Only the animals with glucose levels higher than 16.7 mmol/L were considered diabetic and selected for further studies. One week later, diabetic rats were randomly assigned into three groups (n = 10): group DM treated with metformin 300 mg/(kg • d), group GLY injected with glyburide 5 mg/(kg • d) for 8 weeks.
Care, use, and treatment of all animals in this study were in strict agreement with the rules in the care and use of laboratory animals set forth by Anhui Provincial Hospital Affiliated to Anhui Medical University. Every effort was made to reduce the number of animals used and their suffering.
Biochemical detections
Biochemical detections were approached as described previously [9]. Urine samples were collected from the rats housed in metabolic cages for 24 h to measure urinary albumin (Ualb), urinary sediment PEDF (Upedf), and urinary creatinine (Ucr). Ualb was measured by radioimmunoassay. Radioimmunoassay was used to test Ualb. Enzyme-linked immunosorbent assay was used to test Upedf. Ucr was tested by Jaffe’s assay. Urinary albumin and urinary sediment PEDF were expressed as urinary Ualb/Ucr (UACR) and Upedf/Ucr (UPCR) to eliminate the impact of urine volume, respectively.
Data’s were calculated using the following equations:
UACR (mg/g) = Ualb (mg/L) ÷ [Ucr (umol/L) × the molecular weight of Cr (113.12 g/mol) × 10–6].
UPCR (pg/g) = Upedf (pg/L) ÷ [Ucr (umol/L) × the molecular weight of Cr (113.12 g/mol) × 10–6].
The blood sample was preserved at -80 after centrifugation for the detection of FBG, HbA1c, serum BUN, TG, and LDL-C. High-performance liquid chromatography was used to detect HbA1c. Insulin (INS) was detected by the radioimmunoassay method. BUN was detected by the urease method. The serum TG, LDL and FBG were detected by automatic microplate reader.
Electron microscopic observation
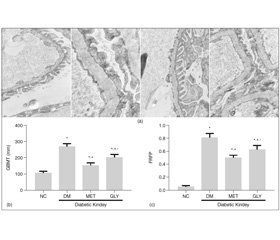
1 mm3 renal cortex was fixed in 2.5 % glutaraldehyde to produce 4 μm thick sections. Ultrathin sections were observed by electron microscopy at a magnification of × 10,000. Three glomeruli were observed per section. Collected 10 photos from each glomerulus randomly and measure glomerular basement membrane (GBM) thickness, taking the average, was calculated as GBMT of the corresponding group.
Image processing and analysis program (Image Pro Plus 6.0) was used to assess the GBM and the part of fused foot process. Foot process fusion rate (FRFP) = the whole length of GBM/the grand total length of fused foot process covered in the corresponding GBM.FRFP was calculated and used to finally assess a mean FRFP per group [10].
Western blot analysis
Western blot analysis was performed as described previously [11]. Briefly, the renal tissue was lysed with the RIPA lysis buffer to obtain extracts of renal protein. Electrophoresis for the protein samples was performed on 10 % SDS-PAGE gels, and then the samples were transferred onto a polyvinylidene fluoride (PVDF) membrane for sealing for 2 h 50 ug of protein from each sample was blotted by an anti-PEDF antibody. The same PVDF membranes were stripped and incubated with an anti-β-actin antibody .Image acquisition system was used to obtain Images, the band intensities were quantified using Image J software.
Renal Tissue PEDF mRNA Expression
The levels of mRNA were analyzed in renal tissue using a real-time PCR approach as described previously [12]. Total RNA was extracted using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. Primers specific for PEDF(forward5'-TCGCATAGACCTTCAGG AGATTA-3',reverse5'-ATCAGAGTCCAAGCCATATCGTA-3'); β-actin (forward5'-CACCCGCGAG TACAACCTTC-3', reverse5'-CCCATACCCACCATCACACC-3').
PCR was performed using the reverse ranscription kit and SYBR Premix Ex Taq II. Results were normalized to the β-actin mRNA levels and represented using the comparative threshold cycle method. The cycle threshold (Ct) value of fluorescence units was used to analyze the mRNA levels by the 7500 software. The relative quantitative method (2−ΔΔCt) was used to calculate the relative changes in gene expression.
Statistical Analysis
Data processing was performed using SPSS 16.0 (SPSS Inc., USA). The results were calculated and expressed as group means ± SD. One-way analysis of variance was used to performed significant differences among groups and Student-Newman-Keuls test was used for comparison between individual groups. Individual differences among groups were analyzed by Dunnett’s test. A p value of < 0.05 was considered statistically significant.
Results
Effect of metformin on serum biochemical and urinary indices in diabetic rats
After 8 weeks, compared with NC group, the diabetic rats were showed a profound elevation in the le–vel of FBG, HbA1c, TG, LDL-C, and BUN and INS (p < 0.05). All measured biochemical parameters above in MET group and GLY group decreased significantly (p < 0.05) as compared with group DM, there were no significant differences in FBG and HbA1c between group MET and group GLY. UACR and UPCR levels in DM group increased obviously compared with NC group. The indicators above in MET group and GLY group were significantly decreased compared to those in the DM group (p < 0.05), there were significantly decreased in group MET than those in group GLY (table 1).
Effect of metformin on renal histology in diabetic rats
The renal tissue obtained from NC group showed a normal mesangial matrix, the structure of glomerular podocytes remained clear, the foot processes showed no fusion. The glomerular basement membrane (GBM) was thickened diffusely and its the architecture became ambiguous, the foot processes were swollen and fused significantly at the meanwhile, some foot processes were completely ruined and even disappeared in the DM group. Compare with DM group, both GBMT and FRFP of glomerular were alleviated in the MET and GLY groups (p < 0.05).Compared to GLY group, the pathological changes of glomerular were more alleviated in MET group (p < 0.05) (fi–gure 1(a)).
Effect of metformin on the renal tissue PEDF protein expressions in diabetic rats
The PEDF protein expressions of the renal tissue in DM group decreased significantly compared to that in NC group (p < 0.05). After intervention with metformin or glimepiride, the expressions of PEDF protein in the renal tissue elevated significantly compared to those in the DM group, there were significantly increased in group MET than those in group GLY (p < 0.05) (fi–gure 2(c)).
Effect of metformin on the renal tissue PEDF mRNA expressions in diabetic rats
As compared to that in NC group, the renal PEDF mRNA expressions was down-regulated significantly in DM group (p < 0.05). Administration of metformin or glyburide significantly inhibited renal PEDF mRNA expressions as compared to DM group, which was more prominent in group MET than GLY (p < 0.05) (figu–re 2(a) (b)).
Discussion
PEDF is a member of the serpin superfamily, which is a neurotrophic factor secreted by adipocytes, and it could block the development and progression of experimental diabetic nephropathy [2]. Initially identified in the conditioned medium of human retinal pigment epithelial cells, it is currently believed that PEDF is abundantly expressed in kidney, liver, and adipose tissue [13].
PEDF has been shown to be a highly effective inhibitor of angiogenesis in cell culture and animal models, not only reduces advanced glycation end product, angiotensin II or leptin-induced vascular endothelial growth factor (VEGF) expression, but also inhibits the biological actions of other factors, thus exerting an important role in protecting blood vessels, inhibiting inflammation and oxidative stress [4, 14, 15]. It was reported that PEDF suppresses AGE-elicited endothelial cell da–mage through its anti-oxidative properties and blocks the progression of experimental diabetic retinopathy (DR) [5, 16].
However, the protective effect of PEDF in early experimental diabetic nephropathy has not been demon–strated in vivo. Joshua [3] provided the first evidence that a PEDF gene delivery ameliorates proteinuria in STZ-induced diabetic rats in vivo. AGE-induced RAGE gene expression, ROS generation, inflammatory and fibrogenic gene expression (MCP-1, TGF-β, fibronectin and type IV collagen mRNA levels) were significantly increased in diabetic kidney, which were suppressed by administration of PEDF, it suggests that PEDF could play a protective role against diabetic nephropathy by attenuating the deleterious effects of AGEs via the downregulation of RAGE expression [5, 18–20].
Previous study showed that metformin treatment increased PEDF expression in both prostate cancer cells and tumor tissue [21]. Turan et al demonstrated that metformin is effective in preventing ovarian hyperstimulation syndrome (OHSS) and its severity (VEGF expression and vascular permeability) by up-regulating PEDF expression [22].
Akın S. et al. reported that patients with newly diagnosed diabetes is associated with obvious increase in serum PEDF levels after metformin treatment [23]. In this study, the levels of serum BUN, UACR, FRFP and GBMT in DM rats were higher than those in group NC. These indexes were significantly decreased after metformin or glyburide treatment for 8 weeks, and the former is superior than the latter, but the value of FBG, HbA1c in MET group had no significant differences compared with GLY group, which indicated that metformin has a remarkable protective effect on the kidney independent of its hypoglycemic effect.
And the underlying mechanisms of metformin reno-protection are not clear completely, which may be related to its effects in activating AMP, activating protein kinase, inhibiting inflammation and oxidative stress, lightening lipid deposition, and so forth [25].
In present study, the UPCR, protein and mRNA expressions of PEDF in group DM were obviously decreased compared with group NC, which were up-regulated by MET or GLY treatment, and that was greater in group MET than that in group GLY. It suggests that metformin can up-regulate the expressions of PEDF protein and mRNA in renal tissue of DM rats partially independent of hypoglycemia, which maybe also contribute to its reno-protection in some extent. At pre–sent, the mechanism is not clear that metformin increasing the expression of PEDF in renal tissue, and there is no report about the direct effect of metformin on the expression and secretion of PEDF in diabetic kidney tissue [23].
In the early stage of diabetes, the expression of PEDF is decreased, hyperglycemia maybe is responsible for the decrease of PEDF in the diabetic kidneys [3, 24]. Many studies have demonstrated that activating PPAR-γ signaling pathway played pivotal role in PEDF’s beneficial effects [25–29]. PPAR-γ knockdown could reduce PEDF expression [30]. The decrease of PEDF was significantly down-regulated and the increase of NF-κB and MMP-9 was down-regulated by systemically admi–nistration of PPAR-γ agonist [31].
Our observations indicate that PEDF induces macrophage apoptosis and necrosis through the signaling of PPAR-γ [25]. Ishibashi et al. demonstrated for the first time that PEDF could block the AGE-induced apoptotic cell death of podocytes by suppressing RAGE expression and subsequent ROS generation partly via PPAR-γ activation. All of the beneficial effects of PEDF on AGE-exposed podocytes were blocked by the treatment of GW9662, an inhibitor of PPAR-γ [32]. PEDF increment on human endothelial cells was blocked by the preincubation with GW9662, which resulted in the promotion of angiogenesis [30]. Moreover, it had been suggested that PEDF could interact with PPAR-γ for the regulation of angiogenesis and lipid metabolism in hepatocellular carcinoma [33], and it is necessary for protective roles of PEDF in OGD cardiomyocytes [34]. These results highlight the crucial of PPAR-γ pathway in regulating PEDF expression.
Previous studies demonstrated that metformin activated AMPK which subsequently activates and upregulates PPAR-γ [35–37]. Qu et al. have shown that metformin can effectively inhibit the mRNA and protein expressions of MCP-1, IL-6, and TNF-α in LPS-induced VSMCs through the upregulation of PPAR-γ activity [38]. Previous studies have also shown that metformin can inhibit LPS-induced pulmonary inflammatory responses in mice by upregulating PPAR-γ activity [39]. In addition, metformin modulates CIS-induced hepatic damage by mitigating oxidative, and decreased caspase-3, MAPK activity and NF-kB level via a PPAR-γ-dependent pathway [40]. These are proposed that metformin increases the PEDF express by up-regulating PPAR-γ pathway, attenuates the deleterious effects of oxidative and fibrogenic, and exerting certain renal protective effects.
Conclusion
Taken together, metformin significantly alleviated proteinuria and renal podocyte lesions in diabetic rats induced by STZ, which may be due to that metformin increases the PEDF express by up-regulating PPAR-γ pathway, attenuates the deleterious effects of oxidative and fibrogenic, and exerting certain renal protective effects.
Acknowledgements
This study was supported by grants from the national natural science foundation (81800713) of China and local scientific and technological development project guided by central government of China (no. 2017070802D147). Considerable help was provided by the department of endocrinology, Anhui provincial hospital affiliated to Anhui medical university.
Conflicts of interests. Authors declare the absence of any conflicts of interests and their own financial interest that might be construed to influence the results or interpretation of their manuscript.
Availability of data and materials
The data’s and models used to support the findings of this study are available from the corresponding author upon request.
- Futrakul N, Butthep P, Futrakul P, Sitprija V. Improvement of renal function in type 2 diabetic nephropathy. Ren Fail. 2007;29(2):155-8. doi: 10.1080/08860220601095835.
- Wang JJ, Zhang SX, Mott R, et al. Anti-inflammatory effects of pigment epithelium-derived factor in diabetic nephropathy. Am J Physiol Renal Physiol. 2008 May;294(5):F1166-73. doi: 10.1152/ajprenal.00375.2007.
- Wang JJ, Zhang SX, Mott R, et al. Salutary Effect of Pigment Epithelium-Derived Factor in Diabetic Nephropathy: Evidence for Antifibrogenic Activities. Diabetes. 2006 Jun;55(6):1678-85. doi: 10.2337/db05-1448.
- Duh EJ, Yang HS, Suzuma I, et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Invest Ophthalmol Vis Sci. 2002 Mar;43(3):821-9.
- Ide Y, Matsui T, Ishibashi Y, Takeuchi M, Yamagishi S. Pigment epithelium-derived factor inhibits advanced glycation end product-elicited mesangial cell damage by blocking NF-κB activation. Microvasc Res. 2010 Sep;80(2):227-32. doi: 10.1016/j.mvr.2010.03.015.
- Ishibashi Y, Matsui T, Takeuchi M, Yamagishi S. Metformin inhibits advanced glycation end products (AGEs)-induced renal tubular cell injury by suppressing reactive oxygen species generation via reducing receptor for AGEs (RAGE) expression. Horm Metab Res. 2012 Nov;44(12):891-5. doi: 10.1055/s-0032-1321878.
- Wendt TM, Tanji N, Guo J, et al. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am J Pathol. 2003 Apr;162(4):1123-37. doi: 10.1016/S0002-9440(10)63909-0.
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998 Sep 12;352(9131):854-65.
- Zhai L, Gu J, Yang D, Wang W, Ye S. Metformin Ameliorates Podocyte Damage by Restoring Renal Tissue Podocalyxin Expression in Type 2 Diabetic Rats. J Diabetes Res. 2015;2015:231825. doi: 10.1155/2015/231825.
- Gundersen HJ, Seefeldt T, Osterby R. Glomerular epithelial foot processes in normal man and rats. Distribution of true width and its intra- and inter-individual variation. Cell Tissue Res. 1980;205(1):147-55. doi: 10.1007/bf00234450.
- Mishra M, Tiwari S, Gomes AV. Protein purification and analysis: next generation Western blotting techniques. Expert Rev Proteomics. 2017 Nov;14(11):1037-1053. doi: 10.1080/14789450.2017.1388167.
- Nolan T, Hands RE, Bustin SA. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 2006;1(3):1559-82. doi: 10.1038/nprot.2006.236.
- Tryggestad JB, Wang JJ, Zhang SX, Thompson DM, Short KR. Elevated plasma pigment epithelium-derived factor in children with type 2 diabetes mellitus is attributable to obesity. Pediatr Diabetes. 2015 Dec;16(8):600-5. doi: 10.1111/pedi.12226.
- Moradi M, Rahimi Z, Amiri S, Rahimi Z, Vessal M, Nasri H. AT1R A1166C variants in patients with type 2 diabetes mellitus and diabetic nephropathy. J Nephropathol. 2015 Jul;4(3):69-76. doi: 10.12860/jnp.2015.14.
- Seki R, Yamagishi S, Matsui T, et al. Pigment epithelium-derived factor (PEDF) inhibits survival and proliferation of VEGF-exposed multiple myeloma cells through its anti-oxidative properties. Biochem Biophys Res Commun. 2013 Feb 22;431(4):693-7. doi: 10.1016/j.bbrc.2013.01.057.
- Yamagishi S, Matsui T, Nakamura K, Takeuchi M, Imaizumi T. Pigment epithelium-derived factor (PEDF) prevents diabetes- or advanced glycation end products (AGE)-elicited retinal leukostasis. Microvasc Res. 2006 Jul-Sep;72(1-2):86-90. doi: 10.1016/j.mvr.2006.04.002.
- Coughlan MT, Thorburn DR, Penfold SA, et al. RAGE-induced cytosolic ROS promote mitochondrial superoxide generation in diabetes. J Am Soc Nephrol. 2009 Apr;20(4):742-52. doi: 10.1681/ASN.2008050514.
- Maeda S, Matsui T, Takeuchi M, et al. Pigment epithelium-derived factor (PEDF) inhibits proximal tubular cell injury in early diabetic nephropathy by suppressing advanced glycation end products (AGEs)-receptor (RAGE) axis. Pharmacol Res. 2011 Mar;63(3):241-8. doi: 10.1016/j.phrs.2010.11.008.
- Yamagishi S, Matsui T, Nakamura K, et al. Pigment-epithelium-derived factor suppresses expression of receptor for advanced glycation end products in the eye of diabetic rats. Ophthalmic Res. 2007;39(2):92-7. doi: 10.1159/000099244.
- Yamagishi S, Matsui T, Nakamura K, Takeuchi M, Imaizumi T. Pigment epithelium-derived factor (PEDF) prevents diabetes- or advanced glycation end products (AGE)-elicited retinal leukostasis. Microvasc Res. 2006 Jul-Sep;72(1-2):86-90. doi: 10.1016/j.mvr.2006.04.002.
- Chen X, Li C, He T, et al. Metformin inhibits prostate cancer cell proliferation, migration, and tumor growth through upregulation of PEDF expression. Cancer Biol Ther. 2016 May 3;17(5):507-14. doi: 10.1080/15384047.2016.1156273.
- Turan GA, Eskicioglu F, Sivrikoz ON, et al. Erratum to: Myo-inositol is a promising treatment for the prevention of ovarian hyperstimulation syndrome (OHSS): an animal study. Arch Gynecol Obstet. 2016 Mar;293(3):691. doi: 10.1007/s00404-016-4021-1.
- Akın S, Aksoy DY, Cınar N, et al. Pigment epithelium-derived factor increases in type 2 diabetes after treatment with metformin. Clin Endocrinol (Oxf). 2012 Dec;77(6):852-6. doi: 10.1111/j.1365-2265.2012.04341.x.
- Chen H, Jia W, Xu X, et al. Upregulation of PEDF expression by PARP inhibition contributes to the decrease in hyperglycemia-induced apoptosis in HUVECs. Biochem Biophys Res Commun. 2008 May 2;369(2):718-24. doi: 10.1016/j.bbrc.2008.02.100.
- Ho TC, Chen SL, Yang YC, et al. PEDF induces p53-mediated apoptosis through PPAR gamma signaling in human umbilical vein endothelial cells. Cardiovasc Res. 2007 Nov 1;76(2):213-23. doi: 10.1016/j.cardiores.2007.06.032.
- Ho TC, Yang YC, Chen SL, et al. Pigment epithelium-derived factor induces THP-1 macrophage apoptosis and necrosis by the induction of the peroxisome proliferator-activated receptor gamma. Mol Immunol. 2008 Feb;45(4):898-909. doi: 10.1016/j.molimm.2007.08.004.
- Ho TC, Chen SL, Shih SC, et al. Pigment epithelium-derived factor (PEDF) promotes tumor cell death by inducing macrophage membrane tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J Biol Chem. 2011 Oct 14;286(41):35943-54. doi: 10.1074/jbc.M111.266064.
- Ho TC, Chen SL, Shih SC, et al. Pigment epithelium-derived factor is an intrinsic antifibrosis factor targeting hepatic stellate cells. Am J Pathol. 2010 Oct;177(4):1798-811. doi: 10.2353/ajpath.2010.091085.
- Wang SH, Liang CJ, Wu JC, et al. Pigment epithelium-derived factor reduces the PDGF-induced migration and proliferation of human aortic smooth muscle cells through PPARγ activation. Int J Biochem Cell Biol. 2012 Feb;44(2):280-9. doi: 10.1016/j.biocel.2011.10.023.
- Lu H, Zhou X, Kwok HH, et al. Ginsenoside-Rb1-Mediated Anti-angiogenesis via Regulating PEDF and miR-33a through the Activation of PPAR-γ Pathway. Front Pharmacol. 2017 Nov 13;8:783. doi: 10.3389/fphar.2017.00783.
- Zhu C, Zhang X, Qiao H, et al. The intrinsic PEDF is regulated by PPARγ in permanent focal cerebral ischemia of rat. Neurochem Res. 2012 Oct;37(10):2099-107. doi: 10.1007/s11064-012-0831-0.
- Ishibashi Y, Matsui T, Ohta K, et al. PEDF inhibits AGE-induced podocyte apoptosis via PPAR-gamma activation. Microvasc Res. 2013 Jan;85:54-8. doi: 10.1016/j.mvr.2012.10.007.
- Chung C, Doll JA, Stellmach VM, et al. Pigment epithelium-derived factor is an angiogenesis and lipid regulator that activates peroxisome proliferator-activated receptor alpha. Adv Exp Med Biol. 2008;617:591-7. doi: 10.1007/978-0-387-69080-3_61.
- Qiu F, Zhang H, Yuan Y, et al. A decrease of ATP production steered by PEDF in cardiomyocytes with oxygen-glucose deprivation is associated with an AMPK-dependent degradation pathway. Int J Cardiol. 2018 Apr 15;257:262-271. doi: 10.1016/j.ijcard.2018.01.034.
- Fu YN, Xiao H, Ma XW, Jiang SY, Xu M, Zhang YY. Metformin attenuates pressure overload-induced cardiac hypertrophy via AMPK activation. Acta Pharmacol Sin. 2011 Jul;32(7):879-87. doi: 10.1038/aps.2010.229.
- Elia EM, Pustovrh C, Amalfi S, Devoto L, Motta AB. Link between metformin and the peroxisome proliferator-activated receptor γ pathway in the uterine tissue of hyperandrogenized prepubertal mice. Fertil Steril. 2011 Jun 30;95(8):2534-7.e1. doi: 10.1016/j.fertnstert.2011.02.004.
- Helmy MM, Helmy MW, El-Mas MM. Additive Renoprotection by Pioglitazone and Fenofibrate against Inflammatory, Oxidative and Apoptotic Manifestations of Cisplatin Nephrotoxicity: Modulation by PPARs. PLoS One. 2015 Nov 4;10(11):e0142303. doi: 10.1371/journal.pone.0142303.
- Qu RN, Qu W. Metformin inhibits LPS-induced inflammatory response in VSMCs by regulating TLR4 and PPAR-gamma. Eur Rev Med Pharmacol Sci. 2019 Jun;23(11):4988-4995. doi: 10.26355/eurrev_201906_18090.
- Ji Y, Wang Z, Li Z, Liu J. Modulation of LPS-mediated Inflammation by Fenofibrate via the TRIF-dependent TLR4 Signaling Pathway in Vascular Smooth Muscle Cells. Cell Physiol Biochem. 2010;25(6):631-40. doi: 10.1159/000315082.
- Mansour HH, El Kiki SM, Galal SM. Metformin and low dose radiation modulates cisplatin-induced oxidative injury in rat via PPAR-γ and MAPK pathways. Arch Biochem Biophys. 2017 Feb 15;616:13-19. doi: 10.1016/j.abb.2017.01.005.

/585-1.jpg)
/586-1.jpg)
/586-2.jpg)