Журнал "Гастроэнтерология" Том 54, №1, 2020
Вернуться к номеру
Assessment of the intestinal microbiota and fecal short-chain fatty acids content in children with non-alcoholic fatty liver disease
Авторы: N.Yu. Zavhorodnia, O.Yu. Lukianenko, I.A. Klenina, O.I. Hrabovska, O.M. Tatarchuk, N.S. Vishnarevska
SI “Institute of Gastroenterology of NAMS of Ukraine”, Dnipro, Ukraine
Рубрики: Гастроэнтерология
Разделы: Клинические исследования
Версия для печати
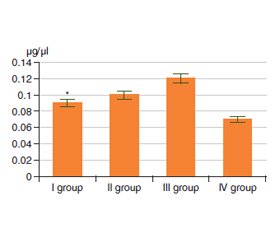
Актуальність. Якісні та кількісні зміни стану кишкового мікробіому сприяють розвитку та прогресуванню неалкогольної жирової хвороби печінки (НАЖХП). Девіації представництва кишкової мікробіоти асоційовані з підвищенням кишкової проникності, активацією механізмів вродженого та адаптивного імунітету, збільшенням продукції та всмоктування коротколанцюгових жирних кислот (КЖК). Співвідношення оцтової, пропіонової, масляної кислот є важливим показником цілісності мікробної спільноти кишечника. Таким чином, вивчення складу мікробіоти кишечника та продукції КЖК є перспективним напрямком, що сприяє розумінню механізмів, які призводять до розвитку НАЖХП у дітей. Мета роботи: вивчити особливості стану кишкової мікрофлори й умісту фекальних коротколанцюгових жирних кислот у дітей з ожирінням з урахуванням наявності НАЖХП. Матеріали та методи. Комплексне обстеження 102 дітей було проведене у відділенні дитячої гастроентерології Інституту гастроентерології Національної академії медичних наук України. Відповідно до наявності ожиріння, даних транзієнтної еластографії і рівня аланінамінотрансферази пацієнти були розділені на чотири групи: I група — діти з простим стеатозом печінки (n = 24); II група — діти з неалкогольним стеатогепатитом (НАСГ) (n = 14); III група — діти з ожирінням без стеатозу печінки (n = 48); IV група — діти з нормальною вагою (n = 16). Хроматографічне дослідження КЖК проводилося за допомогою газового хроматографу «Хроматек-Кристал 5000». Ідентифікація мікроорганізмів здійснювалася з використанням мікробіологічного дослідження вмісту товстої кишки. Діагноз НАЖХП підтверджений за допомогою апарата «FibroScan 502 Touch» (Echosens, Франція) з визначенням контрольованого параметра загасання ультразвуку. Результати. У дітей з ожирінням (III група) виявлені значні зміни в спектрі КЖК: збільшення вмісту оцтової кислоти в 4,8 раза (р < 0,05), пропіонової кислоти в 1,5 раза (р < 0,001) і масляної кислоти в 1,7 раза порівняно з контрольною групою. У дітей з НАСГ вміст оцтової кислоти в копрофільтраті був більше в 2,5 раза, пропіонової та масляної кислот у 1,4 раза порівняно з контрольною групою (р = 0,1). Також спостерігалося значне зниження анаеробного індексу в пацієнтів із НАЖХП. Мікробіологічне дослідження калу продемонструвало зниження кількості біфідобактерій в 11,8 % пацієнтів I групи та у 8,3 % III групи; зниження вмісту Lactobacillus у 70,6 % дітей I групи, у всіх дітей з НАСГ, у 70,8 % пацієнтів III групи. Надмірне зростання Klebsiella виявлене в 23,5 % пацієнтів I групи та у 8,3 % дітей III групи. Патогенний стафілокок знайдений у 5,9 % пацієнтів I групи, у 8,3 % пацієнтів III групи. Надмірне зростання рівня Candida виявлене в 23,5 % дітей I групи, у 14,3 % дітей II групи й у 20,8 % дітей III групи. Висновки. У дітей iз НАЖХП та ожирінням спостерігаються кількісні та якісні зміни кишкової мікробіоти у вигляді зменшення чисельності основних симбіонтів і збільшення представництва умовно-патогенної мікрофлори. Найбільш виражені зміни спектра фекальних КЖК були виявлені в дітей, які страждають від ожиріння, що може свідчити про значущість порушень мікрофлори кишечника на ранніх стадіях розвитку НАЖХП. Визначення співвідношення фракцій фекальних КЖК з обчисленням анаеробного індексу може бути корисним для оцінки стану кишкової мікрофлори в дітей з НАЖХП.
Актуальность. Качественные и количественные изменения кишечного микробиома способствуют развитию и прогрессированию неалкогольной жировой болезни печени (НАЖБП). Девиации представительства кишечной микрофлоры ассоциированы с повышением кишечной проницаемости, активацией врожденных и адаптивных иммунных реакций, увеличением продукции и всасывания в кишечнике короткоцепочечных жирных кислот (КЖК). Соотношение уксусной, пропионовой и масляной кислот является важным показателем целостности микробного сообщества кишечника. Таким образом, изучение состава кишечной микробиоты и продукции короткоцепочечных жирных кислот представляет собой перспективное направление, способствующее пониманию механизмов, приводящих к развитию НАЖБП у детей. Цель работы: изучить особенности состояния кишечной микрофлоры и содержания фекальных короткоцепочечных жирных кислот у детей с НАЖБП. Материалы и методы. Комплексное обследование 102 детей было проведено в отделении детской гастроэнтерологии Института гастроэнтерологии Национальной академии медицинских наук Украины. В зависимости от наличия ожирения, данных транзиентной эластографии и уровня аланинаминотрансферазы пациенты были разделены на четыре группы: I группа — дети с простым стеатозом печени (n = 24); II группа — дети с неалкогольным стеатогепатитом (НАСГ) (n = 14); III группа — дети с ожирением без стеатоза (n = 48), IV группа — дети с нормальным весом (n = 16). Хроматографическое исследование КЖК проводилось с помощью газового хроматографа «Хроматек-Кристалл 5000». Идентификация микроорганизмов осуществлялась с использованием микробиологического исследования содержимого толстой кишки. Диагноз НАЖБП подтвержден при помощи аппарата «FibroScan 502 Touch» (Echosens, Франция) с определением контролируемого параметра затухания ультразвука. Результаты. У детей с ожирением (III группа) выявлены значительные изменения в спектре КЖК: увеличение содержания уксусной кислоты в 4,8 раза (р < 0,05), пропионовой кислоты в 1,5 раза (р < 0,001) и масляной кислоты в 1,7 раза в сравнении с контрольной группой. У детей с НАСГ содержание уксусной кислоты в копрофильтрате было увеличено в 2,5 раза, пропионовой и масляной кислот в 1,4 раза по сравнению с контрольной группой (р = 0,1). Также наблюдалось значительное снижение анаэробного индекса у пациентов с НАЖБП. Микробиологическое исследование кала продемонстрировало снижение количества бифидобактерий у 11,8 % пациентов I группы и у 8,3 % III группы; снижение содержания Lactobacillus у 70,6 % детей I группы, у всех детей с НАСГ, у 70,8 % пациентов III группы. Избыточный рост Klebsiella обнаружен у 23,5 % пациентов I группы и у 8,3 % детей III группы. Патогенный стафилококк выявлен у 5,9 % пациентов I группы, у 8,3 % пациентов III группы. Избыточный рост Candida был выявлен у 23,5 % детей I группы, у 14,3 % детей II группы и у 20,8 % детей III группы. Выводы. У детей с НАЖБП и ожирением наблюдаются количественные и качественные изменения кишечной микробиоты в виде уменьшения численности основных симбионтов и увеличения представительства условно-патогенной микрофлоры. Наиболее выраженные изменения спектра фекальных КЖК были обнаружены у детей, страдающих ожирением, что может свидетельствовать о значимости нарушений микрофлоры кишечника на ранних стадиях развития НАЖБП. Определение соотношения фракций фекальных КЖК с вычислением анаэробного индекса может быть полезным для оценки состояния кишечной микрофлоры у детей с НАЖБП.
Background. Changes in the intestinal microbiome trigger the development and progression of non-alcoholic fatty liver disease (NAFLD). Adverse fluctuations in intestinal microbiota are associated with increased intestinal permeability, activation of mucosal and adaptive immunity, increase in production and intestinal absorption of short-chain fatty acids (SCFA). The ratio of acetic, propionic, butyric acid is an important indicator of the integrity of the microbial community of the intestine. Thus, the study of the gut microbiota composition and short-chain fatty acids production represents a very appealing approach to increasing our knowledge about the mechanisms leading to NAFLD in children. The purpose of the study was to determine the features of the fecal short-chain fatty acids (fSCFA) content and the colonic microbiota composition in children with NAFLD. Materials and methods. A comprehensive examination of 102 children was provided in the Department of Pediatric Gastroenterology of the Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine. According to the presence of obesity, transient elastography data and alanine aminotransferase levels the patients were divided into four groups: I group — children with simple hepatic steatosis (n = 24); II group — children with nonalcoholic steatohepatitis (NASH) (n = 14); III group — children with obesity without steatosis (n = 48), IV group — children with normal weight (n = 16). Chromatographic study of fSCFA was conducted using gas chromatograph Chromatec-Crystal 5000. The microorganisms were identified using a microbiological study of the colon content. Diagnosis of NAFLD was established with FibroScan 502 Touch (Echosens, France) with the determination of the controlled attenuation parameter. Results. Significant changes in the spectrum of fSCFA were observed in children of the III group with acetic acid content increased by 4.8 times (р < 0.05), propionic acid by 1.5 times (р < 0.001), and butyric acid by 1.7 times as compared to the control group, while in children with NASH, acetic content was 2.5-fold increased, propionic and butyric acid — 1.4-fold in comparison with the control group (p = 0.1). Also, significant anaerobic index decrease was observed in NAFLD patients. The fecal content microbiological examination demonstrated the reduced level of Bifidobacteria strains in 11.8 % patients of group I and in 8.3 % of group III; decreased levels of Lactobacillus were found in 70.6 % children of group I, in all children with NASH, in 70.8 % patients of group III. Overgrowth of bacteria such as Klebsiella was identified in 23.5 % patients of group I and in 8.3 % people of group III. Pathogenic Staphylococcus was detected in 5.9 % patients of group I, in 8.3 % patients of group III. Overgrowth of Candida was detected in 23.5 % children of group I, in 14.3 % children of group II and in 20.8 % children of group III. Conclusions. Quantitative and qualitative deviation of intestinal microbiota such as a decrease in the number of major symbionts and an increase in the number of opportunistic microflora was observed in children with NAFLD and obesity. Changes in the SCFA spectrum were found in obese children assuming the importance of intestinal microflora disorders at the early stages of NAFLD development. The estimation of the ratio of SCFA fractions with the anaerobic index calculation can be useful to diagnose intestinal dysbiosis in children with NAFLD.
неалкогольна жирова хвороба печінки; коротколанцюгові жирні кислоти; кишкова мікрофлора; діти; ожиріння
неалкогольная жировая болезнь печени; короткоцепочечные жирные кислоты; кишечная микрофлора; дети; ожирение
non-alcoholic fatty liver disease; short-chain fatty acids; intestinal microflora; children; obesity
Introduction
Materials and methods
Results and discussion
Conclusions
1. Abarca-Gómez L., Abdeen Z.A., Hamid Z.A., Abu-Rmeileh N.M., Acosta-Cazares B., Acuin C., Agyemang C. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128,9 million children, adolescents, and adults. NCD Risk Factor Collaboration (NCD-RisC). Lancet. 2017 Dec 16. 390(10113). 2627-2642. doi: 10.1016/S0140-6736(17)32129-3.
2. Nobili V., Alisi A., Valenti L., Miele L., Feldstein A.E., Alkhouri N. NAFLD in children: new genes, new diagnostic modalities and new drugs. Nat. Rev. Gastroenterol. Hepatol. 2019 Sep. 16(9). 517-530. doi: 10.1038/s41575-019-0169-z.
3. Sharma V., Coleman S., Nixon J., Sharples L., Hamilton-Shield J., Rutter H., Bryant M. A systematic review and meta-analysis estimating the population prevalence of comorbidities in children and adolescents aged 5 to 18 years. Obes. Rev. 2019 Oct. 20(10). 1341-1349. doi: 10.1111/obr.12904.
4. Fang Y.L., Chen H., Wang C.L., Liang L. Pathogenesis of non-alcoholic fatty liver disease in children and adolescence: From “two hit theory” to “multiple hit model”. World J. Gastroenterol. 2018. 24(27). 2974-2983. doi: 10.3748/wjg.v24.i27.2974.
5. Mirza N., Phan T.L., Tester J., Fals A., Fernandez C., Datto G., Estrada E., Eneli I.A. Narrative Review of Medical and Genetic Risk Factors among Children Age 5 and Younger with Severe Obesity. Child. Obes. 2018 Oct. 14(7). 443-452. doi: 10.1089/chi.2017.0350.
6. Greber-Platzer S., Thajer A., Bohn S., Brunert A., Boerner F., Siegfried W., Artlich A., Moeckel A., Waldecker-Krebs H., Pauer S., Holl R.W.; APV-Study Group. Increased liver echogenicity and liver enzymes are associated with extreme obesity, adolescent age and male gender: analysis from the German/Austrian/Swiss obesity registry APV. BMC Pediatr. 2019 Sep 12. 19(1). 332. doi: 10.1186/s12887-019-1711-4.
7. Berardis S., Sokal E. Pediatric non-alcoholic fatty liver disease: an increasing public health issue. Eur. J. Pediatr. 2014. 173. 131-139. doi: 10.1007/s00431-013-2157-6.
8. Wijarnpreecha K., Lou S., Watthanasuntorn K., Kroner P.T., Cheungpasitporn W., Lukens F.J., Pungpapong S., Keaveny A.P., Ungprasert P. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2019 Sep 16. doi: 10.1097/MEG.0000000000001541.
9. Frasinariu O.E., Ceccarelli S., Alisi A., Moraru E., Nobili V. Gut-liver axis and fibrosis in nonalcoholic fatty liver disease: an input for novel therapies. Dig. Liver Dis. 2013 Jul. 45(7). 543-51. doi: 10.1016/j.dld.2012.11.010.
10. Hatton G., Alterio T., Nobili V., Mann J.P. Unmet needs in pediatric NAFLD research: what do we need to prioritize for the future? Expert. Rev. Gastroenterol. Hepatol. 2018 Oct. 12(10). 961-967. doi: 10.1080/17474124.2018.1512853.
11. Schwimmer J.B., Johnson J.S., Angeles J.E., Behling C., Belt P.H., Borecki I., Bross C., Durelle J., Goyal N.P., Hamilton G., Holtz M.L., Lavine J.E., Mitreva M., Newton K.P., Pan A., Simpson P.M., Sirlin C.B., Sodergren E., Tyagi R., Yates K.P., Weinstock G.M., Salzman N.H. Microbiome Signatures Associated with Steatohepatitis and Moderate to Severe Fibrosis in Children with Nonalcoholic Fatty Liver Disease. Gastroenterology. 2019 Oct. 157(4). 1109-1122. doi: 10.1053/j.gastro.2019.06.028.
12. Barrea L., Muscogiuri G., Annunziata G., Laudisio D., Pugliese G., Salzano C., Colao A., Savastano S. From gut microbiota dysfunction to obesity: could short-chain fatty acids stop this dangerous course? Hormones (Athens). 2019 Mar 6. doi: 10.1007/s42000-019-00100-0.
13. Goffredo M., Mass K., Parks E.J. et al. Role of gut microbiota and short chain fatty acids in modulating energy harvest and fat partitioning in youth. J. Clin. Endocrinol. Metab. 2016. 101(11). 4367-4376.
14. Hernández M.A.G., Canfora E.E., Jocken J.W.E., Blaak E.E. The Short-Chain Fatty Acid Acetate in Body Weight Control and Insulin Sensitivity. Nutrients. 2019 Aug 18. 11(8). pii: E1943. doi: 10.3390/nu11081943.
15. Ardatskaya M.D., Garushyan G.V., Moysak R.P., Topchiy T.B. The role of short-chain fatty acids in assessing the state of intestinal microbiocenosis and its correction in patients with NAFLD of various stages. Experimental and clinical gastroenterology. 2019. 161(1). 106-116. doi: 10.31146/1682-8658-ecg-161-1-106-116.
16. Michail S., Lin M., Frey M.R., Fanter R., Paliy O., Hilbush B., Reo N.V. Altered gut microbial energy and metabolism in children with non-alcoholic fatty liver disease. FEMS Microbiol. Ecol. 2015 Feb. 91(2). 1-9. doi: 10.1093/femsec/fiu002.
17. Alyoshkin V.A., Selkova E.P., Zatevalov A.M., Mironov A.Yu., Volchetsky A.L., Gudova N.V. Determination of dysbiotic changes in the gastrointestinal tract by markers of intestinal contents. Federal clinical guidelines. N.-Novgorod: Publishing House “Remediium Volga Region”, 2016. 40 p.
18. Guohua Zhao. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomedical chromatography. 2006. 20. 8. 675-682.
19. Adamberg K., Adamberg S., Ernits K., Larionova A., Voor T., Jaagura M., Visnapuu T., Alamäe T. Composition and metabolism of fecal microbiota from normal and overweight children are differentially affected by melibiose, raffinose and raffinose-derived fructans. Anaerobe. 2018 Aug. 52. 100-110. doi: 10.1016/j.anaerobe.2018.06.009.
20. Murugesan S., Nirmalkar K., Hoyo-Vadillo C., García-Espitia M., Ramírez-Sánchez D., García-Mena J. Gut microbiome production of short-chain fatty acids and obesity in children. Eur. J. Clin. Microbiol. Infect. Dis. 2018. 37(4). 621-625.
21. Miele L., Marrone G., Lauritano С. et al. Gut-liver axis and microbiota in NAFLD: insight pathophysiology for novel therapeutic target. Curr. Pharm. Des. 2013. 19(29). 5314-24.
22. Murugesan S., Ulloa-Martínez M., Martínez-Rojano H. et al. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur. J. Clin. Microbiol. Infect. Dis. 2015. 34(7). 1337-46.

/70.jpg)
/71.jpg)
/71_2.jpg)