Международный эндокринологический журнал Том 16, №5, 2020
Вернуться к номеру
Reasons for the prescription of natural remedies in the treatment of autoimmune thyroiditis with different degrees of hypothyroidism
Авторы: N.Ye. Barabash(1), Yе.V. Simonova(1), T.M. Tykhonova(1), O.М. Nechvohlod(2), O.V. Shvets(3)
(1) — V.N. Karazin Kharkiv National University, Kharkiv, Ukraine
(2) — Private Enterprise “Consultative Polyclinic”, Kharkiv, Ukraine
(3) — Municipal Non-Profit Enterprise “City Hospital 8” of Zaporizhzhia City Council, Zaporizhzhia, Ukraine
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
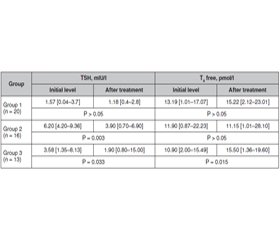
Актуальність. Автоімунний тиреоїдит (АІТ) — найчастіша причина гіпофункції щитоподібної залози. Якщо тактика лікування маніфестного гіпотиреозу шляхом призначення замісної гормональної терапії є доволі однозначною, то питання щодо лікування пацієнтів із субклінічним гіпотиреозом залишається відкритим. Мета: визначення ефективності застосування комбінованого натурального препарату Тіреос при лікуванні АІТ без порушення функції на стадії субклінічного гіпотиреозу як монотерапії і в комплексній терапії маніфестного гіпотиреозу — в комбінації з левотироксином. Матеріали та методи. Обстежено 49 хворих на АІТ віком 48,26 ± 13,52 року, розподілених на три групи. До першої групи залучено 20 хворих на АІТ без порушення функції, до другої групи включено 16 хворих з уперше виявленим субклінічним гіпотиреозом, до третьої групи — 13 хворих, які попередньо отримували терапію L-тироксином з приводу маніфестного гіпотиреозу. Усім пацієнтам було призначено Тіреос по 1 капсулі двічі на добу на період 3 місяці як монотерапію (хворим першої і другої груп) або на додачу до замісної гормональної терапії (хворі третьої групи). Результати. При оцінці скарг виявлено, що через 3 місяці прийому препарату слабкість і швидку втомлюваність відзначали вірогідно менше хворих в усіх трьох групах (р < 0,05). Також вірогідно рідше після тримісячної терапії Тіреосом виявлялася скарга на сухість і випадіння волосся у хворих перших двох груп (р < 0,05). У результаті лікування сухість шкіри вірогідно зменшилась у хворих лише першої обстежуваної групи (р = 0,024). У хворих з уперше виявленим субклінічним гіпотиреозом визначено вірогідну динаміку рівня тиреотропного гормону (ТТГ). У хворих на маніфестний гіпотиреоз додавання Тіреосу до L-тироксину дозволило вірогідно зменшити рівень ТТГ і підвищити показник вільного тироксину без зміни дози замісної гормональної терапії. Усі обстежувані хворі добре переносили прийом препарату за відсутності будь-яких побічних реакцій. Висновки. За наявності субклінічного й маніфестного гіпотиреозу на тлі автоімунного тиреоїдиту цілком виправданим є використання комбінованого натурального засобу Тіреос. Препарат показав клінічно значущий позитивний ефект, що проявлявся в покращенні самопочуття й показників гормонального статусу хворих як у монотерапії при субклінічному гіпотиреозі, так і в комбінації з L-тироксином — при маніфестному. Попередні результати щодо здатності Тіреосу пригнічувати автоімунний процес у щитоподібній залозі потребують подальшого уточнення із залученням більшої кількості хворих і подовженням курсу лікування.
Актуальность. Аутоиммунный тиреоидит является наиболее частой причиной гипофункции щитовидной железы. Если тактика лечения манифестного гипотиреоза путем назначения заместительной гормональной терапии довольно однозначна, то вопрос по лечению пациентов с субклиническим гипотиреозом остается открытым. Целью данного исследования было определить эффективность использования комбинированного натурального препарата Тиреос при лечении аутоиммунного тиреоидита без нарушения функции на стадии субклинического гипотиреоза в качестве монотерапии и в комплексной терапии манифестного гипотиреоза — в комбинации с L-тироксином. Материалы и методы. Обследовано 49 больных аутоиммунным тиреоидитом в возрасте 48,26 ± 13,52 года, разделенных на три группы. В первую группу включены 20 больных аутоиммунным тиреоидитом без нарушения функции, во вторую группу — 16 больных с впервые выявленным субклиническим гипотиреозом, в третью группу — 13 больных, предварительно получавших терапию L-тироксином по поводу манифестного гипотиреоза. Всем пациентам был назначен Тиреос по 1 капсуле два раза в сутки на период 3 месяца в качестве монотерапии (больным первой и второй групп) или в дополнение к заместительной гормональной терапии (больным третьей группы). Результаты. При оценке жалоб выявлено, что через 3 месяца приема препарата слабость и быструю утомляемость отмечали достоверно меньше больных во всех трех группах (р < 0,05). Также достоверно реже после трехмесячной терапии Тиреосом регистрировалась жалоба на сухость и выпадение волос у больных первых двух групп (р < 0,05). В результате лечения сухость кожи доказанно уменьшилась у больных только первой обследуемой группы (р = 0,024). У больных с впервые выявленным субклиническим гипотиреозом определена достоверная позитивная динамика ТТГ. У лиц с манифестным гипотиреозом добавление Тиреоса к терапии L-тироксином позволило существенно снизить уровень тиреотропного гормона (ТТГ) и повысить показатель свободного тироксина без изменения дозы заместительной гормональной терапии. Все обследуемые больные хорошо переносили прием препарата, какие-либо побочные реакции отсутствовали. Выводы. При наличии субклинического и манифестного гипотиреоза на фоне аутоиммунного тиреоидита полностью оправданным является использование комбинированного натурального препарата Тиреос. Он показал клинически значимый положительный эффект, который проявлялся в улучшении самочувствия и показателей гормонального статуса больных как в монотерапии при субклиническом гипотиреозе, так и в комбинации с L-тироксином — при манифестном. Предварительные результаты по эффективности Тиреоса в подавлении аутоиммунного процесса в щитовидной железе требуют дальнейшего уточнения с привлечением большего числа больных и удлинением курса лечения.
Background. Autoimmune thyroiditis is the most common cause of hypothyroidism. If the therapy of overt hypothyroidism by prescribing hormone replacement therapy is quite clear, then the problem of treating the patients with subclinical hypothyroidism remains open. The purpose of this study was to determine the effectiveness of using the combined natural drug Thyreos in the treatment of autoimmune thyroiditis without thyroid dysfunction, at the stage of subclinical hypothyroidism as monotherapy and in the comprehensive therapy of overt hypothyroidism in the combination with L-thyroxine. Materials and methods. Forty-nine patients aged 48.26 ± 13.52 years with autoimmune thyroiditis were examined. All individuals were divided into 3 groups. Group 1 included 20 patients with autoimmune thyroiditis without thyroid dysfunction, group 2 consisted of 16 people with newly diagnosed subclinical hypothyroidism, and group 3 included 13 patients, who previously received L-thyroxine therapy for overt hypothyroidism. All patients were prescribed Thyreos 1 capsule twice a day for a period of 3 months as monotherapy (groups 1 and 2) or in addition to hormone replacement therapy (group 3). Results. When evaluating complaints, it was found that after 3 months of taking the drug, weakness and rapid fatigue were observed in a significantly smaller number of patients in all three groups (p < 0.05). Complaints of dryness and hair loss in individuals of the first two groups (p < 0.05) were also probably less frequent after 3 months of therapy with Thyreos. As a result of treatment, dry skin was significantly reduced in patients of only the first examined group (p = 0.024). In people with newly diagnosed subclinical hypothyroidism, a significant dynamics of thyroid-stimulating hormone level was determined. In patients with overt hypothyroidism, the supplementation of Thyreos to L-thyroxine significantly reduced thyroid-stimulating hormone content and increased free thyroxine level without changing the dose of hormone replacement therapy. All examined individuals tolerated the drug well, without any adverse reactions. Conclusions. In the presence of subclinical and overt hypothyroidism on the background of autoimmune thyroiditis, the use of a combined natural remedy Thyreos is quite justified. The drug showed a clinically significant positive effect, which manifested itself in the improvement of well-being and hormonal status parameters of patients, both as monotherapy in subclinical hypothyroidism and in combination with L-thyroxine — in overt one. Preliminary results of Thyreos effect on the suppression of the autoimmune process in the thyroid gland need further clarification with the involvement of more patients and extension of treatment.
щитоподібна залоза; автоімунний тиреоїдит; гіпотиреоз; лікування; Тіреос
щитовидная железа; аутоиммунный тиреоидит; гипотиреоз; терапия; Тиреос
thyroid gland; autoimmune thyroiditis; hypothyroidism; treatment; Thyreos
Introduction
Material and methods
Results
Discussion
Conclusions
- Chiovato L., Magri F., Carlé A. Hypothyroidism in context: where we’ve been and where we’re going. Adv. Ther. 2019. 36(2). 47-58. DOI: 10.1007/s12325-019-01080-8.
- Wilmar M. Guidance in subclinical hyperthyroidism and subclinical hypothyroidism: are we making progress? Eur. Thyroid J. 2015. 4. 143-148. DOI: 10.1159/000438909.
- Mallipedhi A., Vali H., Okosieme O. Myxedema coma in a patient with subclinical hypothyroidism. Thyroid. 2011. 21(1). DOI: 10.1089/thy.2010.0175.
- Grossman A., Feldhamer I., Meyerovitch J. Treatment with levothyroxin in subclinical hypothyroidism is associated with increased mortality in the elderly. Eur. J. Intern. Med. 2018. 50. 65-68. DOI: 10.1016/j.ejim.2017.11.010.
- Bekkering G.E., Agoritsas T., Lytvyn L. et al. Thyroid hormones treatment for subclinical hypothyroidism: a clinical practice guideline. BMJ. 2019. 365. DOI: https://doi.org/10.1136/bmj.l2006.
- Kottaimuthu R. Ethnobotany of the Valaiyans of Karandamalai, Dindigul District, Tamil Nadu, India. Ethnobotanical Leaflets. 2008. 12. 195-203.
- Bharthi V., Kavya N., Shubhashree M.N., Bhat S. Herbal approach to management of thyroid disease — a review. Journal of Ayurvedic and Herbal Medicine. 2017. 3(1). 51-55.
- Neupane N., Kaur M., Prabhakar Р.K. Treatment of Hashimoto’s thyroiditis with herbal medication. International Journal of Green Pharmacy. 2017. 11(3). 343-347. DOI: 10.22377/ijgp.v11i03.1140.
- Kar A., Bharti S. Relative efficacy of three medicinal plant extracts in the alteration of thyroid hormone concentrations in male mice. Journal of Ethnopharmacology. 2002. 81(2). 281-285. DOI: 10.1016/s0378-8741(02)00048-x.
- Tripathi S., Pathak A.K., Choudhary M., Joshi D.K., Mahdi A.A. Bacopa monneira (brahmi) modulate altered thyroid hormones in Aluminum induced alzheimer’s like rat model. Int. Res J. Pharm. App. Sci. 2013. 3(2). 51-53. URL: www.irjpas.com.
- Stansbury J., Saunders P., Winton D. Promoting health thyroid function with iodine, bladderwrack, guggul and iris. J. Restorative Med. 2012. 1. 83-90.
- Каминский А.В., Киселева И.А., Теплая Е.В. Клинические возможности применения лапчатки белой в профилактике и лечении больных с патологией щитовидной железы. Врачебное дело. 2013. 8. 99-108.
- Ventura M., Melo M., Carrilho F. Selenium and thyroid disease: from pathophysiology to treatment. Int. J. Endocrinol. 2017. 1297658. DOI: 10.1155/2017/1297658.
- Drutel A., Francoise A., Philippe C.D. Selenium and the thyroid gland: more good news for clinicians. Clin. Endocrinol. 2013. 78(2). 155-164. DOI: 10.1111/cen.12066.
- Гончарова О.А. Селен и щитовидная железа (обзор литературы и данные собственных исследований). Ендокринологія. 2014. 19(2). 149-155.
- Guastamacchia E., Giagulli V.A., Licchelli B., Triggiani V. Selenium and iodine in autoimmune thyroiditis. Endocr. Metab. Immune Disord. Drug Targets. 2015. 15(4). 288-92. DOI: 10.2174/1871530315666150619094242.
- Duntas L.H. The role of iodine and selenium in autoimmune thyroiditis. Horm. Metab. Res. 2015. 47(10). 721-6. DOI: 10.1055/s-0035-1559631.
- Гончарова О.А., Ильина И.М. Селенодефицит и возрастозависимая патология (в фокусе дейодиназы). Международный эндокринологический журнал. 2015. 4(68). 87-92.
- Шестакова Т.П. Использование селена в медицинской практике. РМЖ. 2017. 22. 1654-1659.

/57.jpg)