Международный эндокринологический журнал Том 16, №6, 2020
Вернуться к номеру
Prognostic significance of risk factors for thyrotoxicosis development in radioiodine therapy
Авторы: Ubaydullaeva N.B., Allayarova G.I., Almuradov F.F.
Republican Specialized Scientific and Practical Medical Center of Endocrinology named after academician Yo.Kh. Turakulov Ministry of Health of the Republic of Uzbekistan, Tashkent, Republic of Uzbekistan
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
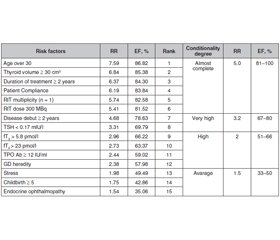
Актуальність. Хвороба Грейвса — системне автоімунне захворювання, яке розвивається внаслідок вироблення антитіл до рецептора тиреотропного гормона, що клінічно проявляється дифузними структурними змінами щитоподібної залози з розвитком синдрому тиреотоксикозу, а також поєднується з екстратиреоїдними проявами. Метою дослідження було виявлення факторів ризику рецидиву хвороби Грейвса в жінок, які отримували терапію радіоактивним йодом. Матеріали та методи. Під спостереженням перебували 93 жінки репродуктивного віку, які отримували радіойодотерапію (РЙТ). Ми проаналізували результати анкетування, оцінили фактори ризику рецидиву тиреотоксикозу і провели ретроспективний і проспективний аналіз клінічних показників і даних анамнезу. Середній вік пацієнтів становив 36,9 ± 7,1 року. Контрольну групу становили 35 здорових жінок віком 33,5 ± 7,6 року. Рівні тиреотропного гормона, вільного трийодотироніну, вільного тироксину й антитіл до тиреоїдної пероксидази визначали імунохемілюмінесцентним методом. Результати. Щоб оцінити якість прогностичної моделі рецидиву тиреотоксикозу, ми розрахували всі параметри факторів ризику AUC. Найбільш значущими факторами ризику розвитку рецидиву тиреотоксикозу після РЙТ із високим відносним ризиком були вік понад 30 років (ВР = 7,59; EF = 8,82 %), обсяг щитоподібної залози ≥ 30 см3 (ВР = 6,84; EF = 85,38 %), тривалість лікування ≥ 2 років (ВР = 6,37; EF = 84,30 %), комплаєнтність пацієнта (ВР = 6,19; EF = 83,84 %), кількість процедур РЙТ (ВР = 5,74; EF = 82,58%) і доза РЙТ 300 МБк (ВР = 5,41; EF = 81,52 %). Висновки. Установлено, що вік понад 30 років (AUC — 0,88), обсяг щитоподібної залози ≥ 30 см3 (AUC — 0,86), тривалість лікування ≥ 2 років (AUC — 0,86), комплаєнтність пацієнта (AUC — 0,85), число процедур РЙТ (AUC — 0,85) і доза I131 (AUC — 0,82) мають першорядне значення.
Актуальность. Болезнь Грейвса — системное аутоиммунное заболевание, развивающееся в результате выработки антител к рецептору тиреотропного гормона, что клинически проявляется диффузными структурными изменениями щитовидной железы с развитием синдрома тиреотоксикоза, а также сочетается с экстратиреоидными проявлениями. Целью исследования было выявление факторов риска рецидива болезни Грейвса у женщин, получавших терапию радиоактивным йодом. Материалы и методы. Под наблюдением находились 93 женщины репродуктивного возраста, получавшие радиойодотерапию (РЙТ). Мы проанализировали результаты анкетирования, оценили факторы риска рецидива тиреотоксикоза и провели ретроспективный и проспективный анализ клинических показателей и данных анамнеза. Средний возраст пациентов составил 36,9 ± 7,1 года. Контрольную группу составили 35 здоровых женщин в возрасте 33,5 ± 7,6 года. Уровни тиреотропного гормона, свободного трийодотиронина, свободного тироксина и антител к тиреоидной пероксидазе определяли иммунохемилюминесцентным методом. Результаты. Чтобы оценить качество прогностической модели рецидива тиреотоксикоза, мы рассчитали все параметры факторов риска AUC. Наиболее значимыми факторами риска развития рецидива тиреотоксикоза после РЙТ с высоким относительным риском были возраст старше 30 лет (ОР = 7,59; EF = 8,82 %), объем щитовидной железы ≥ 30 см3 (ОР = 6,84; EF = 85,38 %), продолжительность лечения ≥ 2 лет (ОР = 6,37; EF = 84,30 %), комплайентность пациента (ОР = 6,19; EF = 83,84 %), количество процедур РЙТ (ОР = 5,74; EF = 82,58 %) и доза РЙТ 300 МБк (ОР = 5,41; EF = 81,52 %). Выводы. Установлено, что возраст старше 30 лет (AUC — 0,88), объем щитовидной железы ≥ 30 см3 (AUC — 0,86), продолжительность лечения ≥ 2 лет (AUC — 0,86), комплайентность пациента (AUC — 0,85), число процедур РЙТ (AUC — 0,85) и доза I131 (AUC — 0,82) имеют первостепенное значение.
Background. Graves’ disease (GD) is a systemic autoimmune disease that develops as a result of the production of antibodies to the thyroid-stimulating hormone receptor, which is clinically manifested by diffuse structural changes in the thyroid gland with the development of thyrotoxicosis syndrome, and also combined with extrathyroid manifestations. The purpose of the study was to identify Graves’ disease recurrence risk factors in women received radioiodine therapy. Materials and methods. 93 women of reproductive age who received radioiodine therapy (RIT) were under obsewrvation. We analyzed the results of the questionnaire and assessed the thyrotoxicosis recurrence risk factors with performed retrospective and prospective analysis of clinical and medical history indicators. Patient’s average age was 36.9 ± 7.1 years. The control group included 35 healthy women aged 33.5 ± 7.6 years. Thyroid stimulating hormone (TSH), free triiodothyonine (fT3) and free thyroxine (fT4) and thyroperoxidase antibodies (TPOAb) levels determined by the immunochemiluminescent method. Results. In order to evaluate the quality of a prognostic model of the thyrotoxicosis recurrence we calculated all risk factors parameters of the AUC. The most significant risk factors for the development of thyrotoxicosis recurrence after RTI with a high relative risk and etiological fraction were age over 30 years (RR = 7.59; EF = 8.82 %), thyroid volume ≥ 30 cm3 (RR = 6.84; EF = 85.38 %), treatment duration ≥ 2 years (RR = 6.37; EF = 84.30 %), patient compliance (RR = 6.19; EF = 83.84 %), RIT multiplicity (RR = 5.74; EF = 82.58 %) and a dose of RIT 300 MBq (RR = 5.41; EF = 81.52 %). Conclusions. It was revealed that the age is older than 30 years (AUC — 0.88), thyroid volume ≥ 30 cm3 (AUC — 0.86), treatment duration ≥ 2 years (AUC – 0.86), patient compliance (AUC — 0.85), RIT multiplicity (AUC — 0.85) and the dose of RIT (AUC — 0.82) have excellent predictive power.
хвороба Грейвса; радіойодотерапія; фактори ризику
болезнь Грейвса; радиойодотерапия; факторы риска
Graves’ disease; radioiodine therapy; risk factors
Introduction
Materials and methods
Results
/82.jpg)
Discussion
Conclusions
- Prasek K., Płazińska M.T., Królicki L. Diagnosis and treatment of Graves’ disease with particular emphasis on appropriate techniques in nuclear medicine. General state of knowledge. Nucl. Med. Rev. Cent. East Eur. 2015. 18. 110-116. doi: 10.5603/NMR.2015.0026.
- Fanning E., Inder W., Mackenzie E. Radioiodine treatment for Graves’ disease: a 10-year Australian cohort study. BMC Endocr. Disord. 2018. 18. 94. doi: 10.1186/s12902-018-0322-7.
- Langenstein C., Schork D., Badenhoop K., Herrmann E. Relapse prediction in Graves´ disease: Towards mathematical modeling of clinical, immune and genetic markers. Rev. Endocr. Metab. Disord. 2016. 17. 571-581. doi: 10.1007/s11154-016-9386-8.
- Ma C., Xie J., Wang H., Wang H., Li J., Chen S. Radioiodine therapy versus antithyroid medications for Graves’ disease. Cochrane Database Syst. Rev. 2016. 2. CD010094. doi: 10.1002/14651858.CD010094.pub2.
- Wang J., Qin L. Radioiodine therapy versus antithyroid drugs in Graves’ disease: a meta-analysis of randomized controlled trials. Br. J. Radiol. 2016. 89(1064). 20160418. doi: 10.1259/bjr.20160418.
- El-Kareem M., Derwish W., Moustafa H. Response rate and factors affecting the outcome of a fixed dose of RAI-131 therapy in Graves’ disease: a 10-year Egyptian experience. Nuclear Medical Community. 2014. 35. 900-907. doi: 10.1097/MNM.0000000000000152.
- Manohar K., Mittal B., Bhoil A., Bhattacharya A., Dutta P., Bhansali A. Factors Predicting Treatment Failure in Patients Treated with Iodine-131 for Graves’ Disease. World J. Nucl. Med. 2013. 12. 57-60. doi: 10.4103/1450-1147.136693.
- Ross D., Burch H., Cooper D., Greenlee M., Laurberg P. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016. 26. 1343-1421. doi: 10.1089/thy.2016.0229.
- Lewis A., Atkinson B., Bell P., Courtney H., McCance D., Mullan K., Hunter S. Outcome of 131I therapy in hyperthyroidism using a 550MBq fixed dose regimen. Ulster Med. J. 2013. 82(2). 85-88. PMID: 24082285; PMCID: PMC3756864.
- Xing Y., Zhang K., Jin G. Predictive factors for the outcomes of Graves’ disease patients with radioactive iodine (131I) treatment. Biosci. Rep. 2020. 40. BSR20191609. doi: 10.1042/BSR20191609.
- Liu M., Jing D., Hu J., Yin S. Predictive factors of outcomes in personalized radioactive iodine ((131)I) treatment for Graves’ disease. Am. J. Med. Sci. 2014. 348. 288-293. doi: 10.1097/MAJ.0000000000000288.

/81.jpg)