Международный эндокринологический журнал Том 16, №8, 2020
Вернуться к номеру
Фізична активність як нефармакологічна та ефективна стратегія в контролі симптомів апоптозу у щурів з експериментальним діабетом
Авторы: M. Bostani, S.A. Noaein
Department of Physical Education, Ahvaz Branch, Islamic Azad University, Ahvaz, Iran
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
Актуальність. За останні роки цукровий діабет (ЦД) став глобальною проблемою охорони здоров’я. Апоптоз бета-клітин підшлункової залози відіграє важливу роль у патогенезі ЦД 1-го типу. Фізична активність як нефармакологічна стратегія для зменшення частоти ускладнень, спричинених ЦД, завжди цікавила дослідників. Тому метою цього дослідження було встановлення впливу аеробних вправ на рівні Bax, Bcl-2 та Bax/Bcl-2 у тканині підшлункової залози стрептозоцин-індукованих діабетичних щурів. Матеріали та методи. Самців щурів лінії Wistar (віком 10 тижнів, вага 200–250 г) загальною кількістю 40 рандомізовано розподілили на групи здорового контролю (ЗК), здорових тренованих (ЗT), з діабетом (контроль, ДК) та з діабетом із фізичним навантаженням (ДН). Діабет був індукований одноразовою інтраперитонеальною ін’єкцією стрептозоцину (45 мг/кг). Тренувальні групи виконували вправу на біговій доріжці п’ять днів поспіль протягом шести тижнів. Рівні тканин підшлункової залози в білках Bax та Bcl-2 додатково визначали методом ІФА. Результати. Індукція діабету суттєво знизила рівень білка Bcl-2 та збільшила рівень білка Bax та співвідношення Bax/Bcl-2 у тканині підшлункової залози (p < 0,05). Окрім того, результати показали, що шість тижнів аеробних тренувань значно підвищили рівень білка Bcl-2 та знизили рівень білка Bax у групі ДН. Також співвідношення Bax/Bcl-2 суттєво знизилось у групі ДН (р < 0,05). Посилення виділення та передачі фактора, що індукує апоптоз, який спостерігається в стані окислювального стресу, зменшується в тканинах тренованих осіб, що вказує на гальмування в сигналізації апоптозу. Висновки. Згідно з результатами цього дослідження, фізичні вправи можна розглядати як ефективну стратегію зменшення частоти спричиненого діабетом апоптозу та контролю його ускладнень.
Актуальность. За последние годы сахарный диабет (СД) стал глобальной проблемой здравоохранения. Апоптоз бета-клеток поджелудочной железы играет важную роль в патогенезе СД 1-го типа. Физическая активность как нефармакологическая стратегия для уменьшения частоты осложнений, вызванных диабетом, всегда интересовала исследователей. Поэтому целью данного исследования было установление влияния аэробных упражнений на уровни Bax, Bcl-2 и Bax/Bcl-2 в ткани поджелудочной железы стрептозоцин-индуцированных диабетических крыс. Материалы и методы. Самцы крыс линии Wistar (в возрасте 10 недель, вес 200–250 г), общее количество 40, были рандомизированы на группы здорового контроля (ЗК), здоровых тренированных (ЗТ), с диабетом (контроль, ДК) и с диабетом с физической нагрузкой (ДН). Диабет был индуцирован однократной интраперитонеальной инъекцией стрептозоцина (45 мг/кг). Тренировочные группы выполняли упражнения на беговой дорожке пять дней подряд в течение шести недель. Уровни тканей поджелудочной железы в белках Bax и Bcl-2 дополнительно определяли методом ИФА. Результаты. Индукция диабета существенно снизила уровень белка Bcl-2 и увеличила уровень белка Bax и соотношение Bax/Bcl-2 в ткани поджелудочной железы (p < 0,05). Кроме того, результаты показали, что шесть недель аэробных тренировок значительно повысили уровень белка Bcl-2 и снизили уровень белка Bax в группе ДН. Также соотношение Bax/Bcl-2 существенно снизилось в группе ДН (р < 0,05). Усиление выделения и передачи фактора, индуцирующего апоптоз, который наблюдается в состоянии окислительного стресса, уменьшается в тканях тренированных лиц и указывает на торможение в сигнализации апоптоза. Выводы. Согласно результатам этого исследования, физические упражнения можно рассматривать как эффективную стратегию уменьшения частоты апоптоза, вызванного диабетом, и контроля его осложнений.
Background. In recent years, diabetes has become a global health problem. Apoptosis of pancreatic beta cells plays an important role in the pathogenesis of type 1 diabetes. Exercise as a non-pharmacological strategy to reduce the diabetic-induced complications has always been of interest to researchers. Therefore, the purpose of this study was to investigate the effect of aerobic exercise on levels of Bax, Bcl-2 and Bax/Bcl-2 ratio in pancreatic tissue of streptozotocin (STZ)-induced diabetic rats. Materials and methods. A total number of 40 male Wistar rats (10 weeks old, 200–250 gr weight) were randomly divided into healthy control (HC), healthy trained (HT), diabetic control (DC), and diabetic trained (DT) groups. Diabetes was also induced by a single intraperitoneally injection of streptozocin (45 mg/kg). The training groups performed the exercise on the treadmill for five consecutive days within six weeks. The pancreatic tissue levels of the Bax and the Bcl-2 proteins were further determined via ELISA method. Results. The results showed that the induction of diabetes had significantly decreased the levels of Bcl-2 protein and increased the levels of Bax protein and Bax/Bcl-2 ratio in the pancreatic tissue (p < 0.05). As well, the findings showed that six weeks of aerobic exercise training had significantly increased the levels of Bcl-2 and significantly decreased the levels of Bax protein in DT group. Also, the Bax/Bcl-2 ratio reduced significantly in DT group (p < 0.05). The increase in displacement and transmission of apoptosis inducing factor (AIF) that have seen in oxidative stress status, is reduced in the tissues of trained individuals which indicating of the inhibition in the apoptotic signaling. Conclusions. According to the results of this study, exercise can be considered as an effective strategy to reduce the rate of diabetic-induced apoptosis and control its complications.
фізичні вправи; Bax; Bcl-2; апоптоз; цукровий діабет
физические упражнения; Bax; Bcl-2; апоптоз; сахарный диабет
exercise; Bax; Bcl-2; apoptosis; diabetes mellitus
Introduction
Materials and methods
Animals
Diabetes induction
Treadmill training protocol
Tissue extraction
Evaluation of Bax and Bcl-2
Statistical analysis
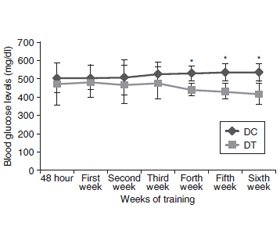
Results
/13.jpg)
Discussion
Conclusions
- Zhao L., Gu Q., Xiang L., Dong X., Li H., Ni J., Wan L., Cai G., Chen G. Curcumin inhibits apoptosis by modulating Bax/Bcl-2 expression and alleviates oxidative stress in testes of streptozotocin-induced diabetic rats. Ther. Clin. Risk Manag. 2017. 13. 1099-1105. doi: 10.2147/TCRM.S141738.
- Atkinson M.A. Thirty years of investigating the autoimmune basis for type 1 diabetes: why can’t we prevent or reverse this disease? Diabetes. 2005. 54(5). 1253-1263. doi: 10.2337/diabetes.54.5.1253.
- Kim K.A., Lee M.S. Recent progress in research on beta-cell apoptosis by cytokines. Front. Biosci (Landmark Ed). 2009. 14. 657-64. doi: 10.2741/3271.
- Kroemer G., Galluzzi L., Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007. 87(1). 99-163. doi: 10.1152/physrev.00013.2006.
- Susnow N., Zeng L., Margineantu D., Hockenbery D.M. Bcl-2 family proteins as regulators of oxidative stress. Semin. Cancer. Biol. 2009. 19(1). 42-9. doi: 10.1016/j.semcancer.2008.12.002.
- Raisova M., Hossini A.M., Eberle J., Riebeling C., Wieder T., Sturm I., Daniel P.T. et al. The Bax/Bcl-2 ratio determines the susceptibility of human melanoma cells to CD95/Fas-mediated apoptosis. J. Invest. Dermatol. 2001. 117(2). 333-40. doi: 10.1046/j.0022-202x.2001.01409.x.
- Youle R., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008. 9. 47-59. https://doi.org/10.1038/nrm2308
- Wattenberg B., Lithgow T. Targeting of C-terminal (tail)-anchored proteins: understanding how cytoplasmic activities are anchored to intracellular membranes. Traffic. 2001. 2(1). 66-71. doi: 10.1034/j.1600-0854.2001.20108.x.
- Asa C., Maria S., Katharina S.S., Bert A. Aquatic exercise is effective in improving exercise performance in patients with heart failure and type 2 diabetes mellitus. Evid. Based Complement. Alternat. Med. 2012. 2012. 349209. doi: 10.1155/2012/349209.
- Selagzi H., Buyukakilli B., Cimen B., Yilmaz N., Erdogan S. Protective and therapeutic effects of swimming exercise training on diabetic peripheral neuropathy of streptozotocin-induced diabetic rats. J. Endocrinol. Invest. 2008. 31(11). 971-8. doi: 10.1007/BF03345634. PMID: 19169052.
- Kadoglou N.P., Vrabas I.S., Kapelouzou A., Lampropoulos S., Sailer N., Kostakis A., Liapis C.D., Angelopoulou N. The impact of aerobic exercise training on novel adipokines, apelin and ghrelin, in patients with type 2 diabetes. Med. Sci Monit. 2012. 18(5). CR290-5. doi: 10.12659/msm.882734.
- Cheng S.M., Ho T.J., Yang A.L., Chen I.J., Kao C.L., Wu F.N., Lin J.A. Exercise training enhances cardiac IGFI-R/PI3K/Akt and Bcl-2 family associated pro-survival pathways in streptozotocin-induced diabetic rats. International Journal of Cardiology. 2012. 167(2). doi: 10.1016/j.ijcard.2012.01.031.
- Ramezani N., Vanaky B., Shakeri N., Fakhari Rad F., Shams Z. Evaluation of Bcl-2 and Bax Expression in the Heart of Diabetic Rats after Four Weeks of High Intensity Interval Training. Medical Laboratory Journal. 2019. 13(1). 15-20. doi: 10.29252/mlj.13.1.15.
- Høydal M.A., Wisloff U., Kemi O.J., Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: Practical implications for exercise training. European Journal of Cardiovascular Prevention and Rehabilitation. 2008. 14(6). 753-60. doi: 10.1097/HJR.0b013e3281eacef1.
- Chae C.H., Jung S.L., An S.H., Park B.Y., Wang S.W., Cho I.H., Cho J.Y., Kim H.T. Treadmill exercise improves cognitive function and facilitates nerve growth factor signaling by activating mitogen-activated protein kinase/extracellular signal-regulated kinase1/2 in the streptozotocin-induced diabetic rat hippocampus. Neuroscience. 2009. 164(4). 1665-73. doi: 10.1016/j.neuroscience.2009.09.075.
- Devine P.J., Perreault S.D., Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012. 86(2). 27. doi: 10.1095/biolreprod.111.095224.
- Agarwal A. et al. The effects of oxidative stress on female reproduction: a review. Reprod. Biol. Endocrinol. 2012. 10. 49. https://doi.org/10.1186/1477-7827-10-49.
- Nayki U., Onk D., Balci G., Nayki C., Onk A., Gunay M. The Effects of Diabetes Mellitus on Ovarian Injury and Reserve: An Experimental Study. Gynecol. Obstet. Invest. 2016. 81(5). 424-9. doi: 10.1159/000442287.
- Gomez-Cabrera M.C., Viña J., Ji L.L. Interplay of oxidants and antioxidants during exercise: implications for muscle health. Phys. Sportsmed. 2009. 37(4). 116-23. doi: 10.3810/psm.2009.12.1749.
- Batista M.L. Jr, Rosa J.C., Lopes R.D., Lira F.S. et al. Exercise training changes IL-10/TNF-α ratio in the skeletal muscle of post-MI rats. Cytokine. 2010. 1(49). 102-108. https://doi.org/10.1016/j.cyto.2009.10.007
- Siu P.M., Bryner R.W., Martyn J.K., Alway S.E. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. FASEB J. 2004. 18(10). 1150-2. doi: 10.1096/fj.03-1291fje.
- Fisher-Wellman K., Bloomer R.J. Acute exercise and oxidative stress: a 30 year history. Dyn. Med. 2009. 8. 1. doi: 10.1186/1476-5918-8-1.
- Dejean L.M., Martinez-Caballero S., Manon S., Kinnally K.W. Regulation of the mitochondrial apoptosis-induced channel, MAC, by BCL-2 family proteins. Biochim. Biophys. Acta. 2006. 1762(2). 191-201. doi: 10.1016/j.bbadis.2005.07.002.
- Peterson J.M., Bryner R.W., Sindler A., Frisbee J.C., Alway S.E. Mitochondrial apoptotic signaling is elevated in cardiac but not skeletal muscle in the obese Zucker rat and is reduced with aerobic exercise. J. Appl. Physiol (1985). 2008. 105(6). 1934-43. doi: 10.1152/japplphysiol.00037.2008.
- Fang J., Wu L., Chen L. Postconditioning attenuates cardiocyte ultrastructure injury and apoptosis by blocking mitochondrial permeability transition in rats. Acta Cardiol. 2008. 63(3). 377-87. doi: 10.2143/AC.63.3.1020316.
- Garcia-Saez A. The secrets of the Bcl-2 family. Cell Death & Differentiation. 2012. 19(11). 1733-1740. doi: 10.1038/cdd.2012.105.
- Kwak H.B. Effects of aging and exercise training on apoptosis in the heart. J. Exerc. Rehabil. 2013. 9(2). 212-9. doi: 10.12965/jer.130002.
- Vainshtein A., Kazak L., Hood D.A. Effects of endurance training on apoptotic susceptibility in striated muscle. J. Appl. Physiol (1985). 2011. 110(6). 1638-45. doi: 10.1152/japplphysiol.00020.2011.
- Bostani M., Rahmati M., Mard S.A. The effect of endurance training on levels of LINC complex proteins in skeletal muscle fibers of STZ-induced diabetic rats. Sci Rep. 2020. 10. 8738. https://doi.org/10.1038/s41598-020-65793-5

/12.jpg)
/13_2.jpg)