Международный эндокринологический журнал Том 16, №8, 2020
Вернуться к номеру
Вплив лікування метформіном на рівень GLP-1, NT-proBNP та ендотеліну-1 у крові хворих на цукровий діабет 2-го типу
Авторы: L.K. Sokolova, Yu.B. Belchina, V.V. Pushkarev, S.A. Cherviakova, T.S. Vatseba, O.I. Kovzun, V.M. Pushkarev, M.D. Tronko
State Institution “V.P. Komisarenko Institute of Endocrinology and Metabolism of the NAMS of Ukraine”,
Kyiv, Ukraine
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
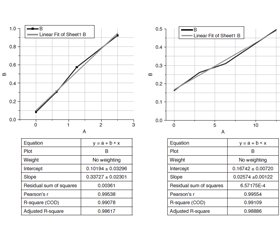
Актуальність. Цукровий діабет 2-го типу (ЦД2) тісно пов’язаний з підвищеним ризиком серцево-судинних захворювань. Було показано, що ендотеліальна дисфункція є однією з ключових патологічних подій у розвитку хронічних судинних ускладнень діабету. Важливим ефектом ендотеліальної дисфункції є те, що вона збільшує продукцію і біологічну активність сильнодіючого вазоконстриктора і прозапального пептиду — ендотеліну (ЕТ). Метформін використовується при лікуванні ЦД2 як препарат першої лінії. Встановлено, що механізм дії метформіну може бути пов’язаний з біохімічними процесами в шлунково-кишковому тракті. Мозковий натрійуретичний пептид (BNP) використовується як маркер при діагностиці серцевої недостатності. Метою цієї роботи було визначення і зіставлення рівнів ET-1, NT-proBNP і глюкагоноподібного пептиду-1 (GLP-1) у крові пацієнтів із ЦД2, які отримували метформін. Матеріали та методи. Концентрації NT-proBNP, GLP-1, ЕТ-1 і глікованого гемоглобіну визначали за допомогою імуноферментного аналізу. Для порівняння груп даних використовували t-критерій Стьюдента і однофакторний дисперсійний аналіз. Результати. Кількість ЕТ-1 у крові хворих на ЦД2 значно перевищує його концентрацію в контрольних зразках. Монотерапія метформіном приводить до зниження рівня ЕТ-1 більше ніж на 65 %. Комбінована терапія метформіном з інсуліном викликає ще більше зменшення кількості ЕТ-1. Рівень GLP-1 у крові хворих на ЦД2 значно, більше ніж удвічі, знижений порівняно зі здоровими людьми. Після лікування метформіном умiст GLP-1 збільшується до контрольного рівня. Кількість NT-proBNP у крові хворих на цукровий діабет перевищує контрольні значення більше ніж удвічі. Лікування метформіном приводить до зниження рівня натрійуретичного пептиду більше ніж на 40 %. Висновки. Таким чином, лікування метформіном обумовлює зниження концентрацій ET-1 і NT-proBNP, а також підвищення рівня GLP-1 у крові пацієнтів із ЦД2. Разом ці події можуть указувати на позитивний захисний ефект метформіну на серцево-судинну систему.
Актуальность. Сахарный диабет 2-го типа (СД2) тесно связан с повышенным риском сердечно-сосудистых заболеваний. Было показано, что эндотелиальная дисфункция является одним из ключевых патологических событий в развитии хронических сосудистых осложнений диабета. Важным эффектом эндотелиальной дисфункции является то, что она увеличивает продукцию и биологическую активность сильнодействующего вазоконстриктора и провоспалительного пептида — эндотелина (ЭТ). Метформин используется при лечении СД2 в качестве препарата первой линии. Установлено, что механизм действия метформина может быть связан с биохимическими процессами в желудочно-кишечном тракте. Мозговой натрийуретический пептид (BNP) используется в качестве маркера при диагностике сердечной недостаточности. Целью данной работы было определение и сопоставление уровней ЭT-1, NT-proBNP и глюкагоноподобного пептида-1 (GLP-1) в крови пациентов с СД2, получавших метформин. Материалы и методы. Концентрации NT-proBNP, GLP-1, ЭТ-1 и гликированного гемоглобина определяли с помощью иммуноферментного анализа. Для сравнения групп данных использовались t-критерий Стьюдента и однофакторный дисперсионный анализ. Результаты. Количество ЭТ-1 в крови больных СД2 значительно превышает его концентрацию в контрольных образцах. Монотерапия метформином приводит к снижению уровня ЭТ-1 более чем на 65 %. Комбинированная терапия метформином и инсулином вызывает еще большее уменьшение количества ЭТ-1. Уровень GLP-1 в крови больных СД2 значительно, более чем в 2 раза, снижен по сравнению со здоровыми людьми. После лечения метформином содержание GLP-1 увеличивается до контрольного уровня. Количество NT-proBNP в крови больных сахарным диабетом превышает контрольные значения более чем в 2 раза. Лечение метформином приводит к снижению уровня натрийуретического пептида более чем на 40 %. Выводы. Таким образом, лечение метформином обусловливает снижение концентраций ЭT-1 и NT-proBNP, а также повышение уровня GLP-1 в крови пациентов с СД2. Вместе эти события могут указывать на положительный защитный эффект метформина на сердечно-сосудистую систему.
Background. Type 2 diabetes mellitus (T2DM) is closely associated with an increased risk of cardiovascular diseases. It was shown that endothelial dysfunction is one of the key pathological events in the development of chronic vascular diabetic complications. An important effect of endothelial dysfunction is that it increases the production and biological activity of the potent vasoconstrictor and the pro-inflammatory peptide — endothelin (ET). Metformin is used in the treatment of T2DM as a first-line medication. It has been shown that the mechanism of action of metformin may be associated with biochemical processes in the gastrointestinal tract. Brain natriuretic peptide (BNP) is used as a marker in the diagnosis of heart failure. The purpose of this work was to determine and compare ET-1, NT-proBNP and glucagon-like peptide-1 (GLP-1) blood levels in diabetic patients treated with metformin. Materials and methods. NT-proBNP, GLP-1, endothelin-1 and glycated hemoglobin were determined using enzyme-linked immunosorbent assay. To compare the data groups, Student’s t-test and one-way ANOVA were used. Results. The content of ET-1 in the blood of patients with T2DM significantly exceeds its concentration in the control samples. Monotherapy with metformin leads to a decrease in ET-1 levels by more than 65 %. The combination therapy of metformin with insulin causes even greater decrease in ET-1. The blood level of GLP-1 in patients with T2DM is significantly, more than 2 times, reduced compared to healthy people. After metformin treatment, the content of GLP-1 is increased to the control level. The concentration of NT-proBNP in the blood of diabetic patients more than 2 times exceeds the control values. Treatment with metformin leads to a decrease in the content of natriuretic peptide by more than 40 %. Conclusions. Thus, treatment with metformin causes a decrease in ET-1 and NT-proBNP concentrations, and an increase in blood GLP-1 of patients with type 2 diabetes. These events together may indicate a positive protective effect of metformin on the cardiovascular system.
цукровий діабет 2-го типу; NT-proBNP; глюкагоноподібний пептид-1; ендотелін-1
сахарный диабет 2-го типа; NT-proBNP; глюкагоноподобный пептид-1; эндотелин-1
type 2 diabetes; NT-proBNP; glucagon-like peptide-1; endothelin-1
Introduction
Materials and methods
Results
Discussion
Conclusions
- Dhananjayan R., Koundinya K.S., Malati T., Kutala V.K. Endothelial dysfunction in type 2 diabetes mellitus. Indian J. Clin. Biochem. 2016. 31(4). 372-9. doi: 10.1007/s12291-015-0516-y.
- Ke J., Liu Y., Yang J., Lu R., Tian Q., Hou W., Wang G., Wei R., Hong T. Synergistic effects of metformin with liraglutide against endothelial dysfunction through GLP-1 receptor and PKA signalling pathway. Sci. Rep. 2017. 7. 41085. doi: 10.1038/srep41085.
- Jain A., Chen S., Yong H., Chakrabarti S. Endothelin-1 traps potently reduce pathologic markers back to basal levels in an in vitro model of diabetes. J. Diabetes Metab. Disord. 2018. 17(2). 189-95. doi: 10.1007/s40200-018-0360-8.
- Johnström P., Fryer T.D., Richards H.K., Maguire J.J., Clark J.C., Pickard J.D., Davenport A.P. Positron emission tomography of [18F]-big endothelin-1 reveals renal excretion but tissue-specific conversion to [18F]-endothelin-1 in lung and liver. Br. J. Pharmacol. 2010. 159. 812-9. doi: 10.1111/j.1476-5381.2010.00641.x.
- Kalani M. The importance of endothelin-1 for microvascular dysfunction in diabetes. Vasc. Health Risk Manag. 2008. 4(5). 1061-8. doi: 10.2147/vhrm.s3920.
- El-Mesallamy H., Suwailem S., Hamdy N. Evaluation of C-reactive protein, endothelin-1, adhesion molecule(s), and lipids as inflammatory markers in type 2 diabetes mellitus patients. Mediators Inflamm. 2007. 2007. 73635. doi: 10.1155/2007/73635.
- McCreight L.J., Bailey C.J., Pearson E.R. Metformin and the gastrointestinal tract. Diabetologia. 2016. 59(3). 426-35. doi: 10.1007/s00125-015-3844-9.
- Bahne E., Sun E.W.L., Young R.L., Hansen M., Sonne D.P., Hansen J.S., Rohde U. et al. Metformin-induced glucagon-like peptide-1 secretion contributes to the actions of metformin in type 2 diabetes. JCI Insight. 2018. 3(23). e93936. doi: 10.1172/jci.insight.93936.
- DeFronzo R.A., Buse J.B., Kim T., Burns C., Skare S., Baron A., Fineman M. Once-daily delayed-release metformin lowers plasma glucose and enhances fasting and postprandial GLP-1 and PYY: results from two randomized trials. Diabetologia. 2016. 59(8). 1645-54. doi: 10.1007/s00125-016-3992-6.
- Wolsk E., Claggett B., Pfeffer M.A., Diaz R., Dickstein K., Gerstein H.C., Lawson F.C. et al. Role of B-type natriuretic peptide and N-terminal prohormone BNP as predictors of cardiovascular morbidity and mortality in patients with a recent coronary event and type 2 diabetes mellitus. J. Am. Heart Assoc. 2017. 6(6). e004743. doi: 10.1161/JAHA.116.004743.
- Mahadavan G., Nguyen T.H., Horowitz J.D. Brain natriuretic peptide: a biomarker for all cardiac disease? Curr. Opin. Cardiol. 2014. 29(2). 160-6. doi: 10.1097/HCO.0000000000000036.
- Baldassarre S., Fragapani S., Panero A., Fedele D., Pinach S., Lucchiari M., Vitale A.R. et al. NTproBNP in insulin-resistance mediated conditions: overweight/obesity, metabolic syndrome and diabetes. The population-based Casale Monferrato Study. Cardiovasc. Diabetol. 2017. 16(1). 119. doi: 10.1186/s12933-017-0601-z.
- Markowicz-Piasecka M., Huttunen K.M., Sadkowska A., Sikora J. Pleiotropic activity of metformin and its sulfonamide derivatives on vascular and platelet haemostasis. Molecules. 2019. 25(1). 125. doi: 10.3390/molecules25010125.
- Davenport A.P. Endothelin. Pharmacol. Rev. 2016. 68(2). 357-418. doi: 10.1124/pr.115.011833.
- Pscherer S., Freude T., Forst T., Nussler A.K., Braun K.F., Ehnert S. Anti-diabetic treatment regulates pro-fibrotic TGF-β serum levels in type 2 diabetics. Diabetol. Metab. Syndr. 2013. 5(1). 48. doi: 10.1186/1758-5996-5-48.
- Xiao H., Zhang J., Xu Z., Feng Y., Zhang M., Liu J., Chen R. et al. Metformin is a novel suppressor for transforming growth factor (TGF)-β1. Sci. Rep. 2016. 6. 28597. doi: 10.1038/srep28597.
- Sokolova L.K., Belchina Yu.B., Pushkarev V.V., Cherviakova S.A., Vatseba T.S., Kovzun O.I., Pushkarev V.M., Tronko M.D. The blood level of endothelin-1 in diabetic patients depending on the characteristics of the disease. Mìžnarodnij endokrinologìčnij žurnal. 2020. 16(3). 204-208. doi: 10.22141/2224-0721.16.3.2020.205267.
- Yang X., Xu Z., Zhang C., Cai Z., Zhang J. Metformin, beyond an insulin sensitizer, targeting heart and pancreatic β cells. Biochim. Biophys. Acta. 2017. 1863(8). 1984-90. doi: 10.1016/j.bbadis.2016.09.019.
- Zhou L., Cai X., Li M., Han X., Ji L. Plasma NT-proBNP is independently associated with albuminuria in type 2 diabetes. J. Diabetes Complications. 2016. 30(4). 669-74. doi: 10.1016/j.jdiacomp.2016.01.017.
- Valentine R.J., Coughlan K.A., Ruderman N.B., Saha A.K. Insulin inhibits AMPK activity and phosphorylates AMPK Ser485/491 through Akt in hepatocytes, myotubes and incubated rat skeletal muscle. Arch. Biochem. Biophys. 2014. 562. 62-9. doi: 10.1016/j.abb.2014.08.013.
- Pushkarev V.V., Sokolova L.K., Pushkarev V.M., Belchina Y.B., Vatseba T.S., Tronko N.D. Effect of combined treatment with insulin and other hypoglycemic drugs on 5'AMP-activated protein kinase activity in lymphocytes in patients with diabetes mellitus. Prob. Endocrin. Pathol. 2019. 3. 74-82. doi: 10.21856/j-PEP.2019.3.10.
- Lteif A., Vaishnava P., Baron A.D., Mather K.J. Endothelin limits insulin action in obese/insulin-resistant humans. Diabetes. 2007. 56(3). 728-34. doi: 10.2337/db06-1406.
- Sarafidis P.A., Bakris G.L. Review: Insulin and endothelin: an interplay contributing to hypertension development? J. Clin. Endocrinol. Metab. 2007. 92(2). 379-85. doi: 10.1210/jc.2006-1819.
- Koska J., Sands M., Burciu C., D’Souza K.M., Raravikar K., Liu J., Truran S. et al. Exenatide protects against glucose- and lipid-induced endothelial dysfunction: evidence for direct vasodilation effect of GLP-1 receptor agonists in humans. Diabetes. 2015. 64(7). 2624-35. doi: 10.2337/db14-0976.
- Lastya A., Saraswati M.R., Suastika K. The low level of glucagon-like peptide-1 (GLP-1) is a risk factor of type 2 diabetes mellitus. BMC Res. Notes. 2014. 7. 849. doi: 10.1186/1756-0500-7-849.
- Napolitano A., Miller S., Nicholls A.W., Baker D., Van Horn S., Thomas E., Rajpal D. et al. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS One. 2014. 9(7). e100778. doi: 10.1371/journal.pone.0100778.
- Zilov A.V., Abdelaziz S.I., AlShammary A., Al Zahrani A., Amir A., Assaad Khalil S.H., Brand K. et al. Mechanisms of action of metformin with special reference to cardiovascular protection. Diabetes Metab. Res. Rev. 2019. 35(7). e3173. doi: 10.1002/dmrr.3173.
- Liu G., Wu K., Zhang L., Dai J., Huang W., Lin L., Ge P. et al. Metformin attenuated endotoxin-induced acute myocarditis via activating AMPK. Int. Immunopharmacol. 2017. 47. 166-72. doi: 10.1016/j.intimp.2017.04.002.
- Loi H., Boal F., Tronchere H., Cinato M., Kramar S., Oleshchuk O., Korda M., Kunduzova O. Metformin protects the heart against hypertrophic and apoptotic remodeling after myocardial infarction. Front. Pharmacol. 2019. 10. 154. doi: 10.3389/fphar.2019.00154.
- Han Y., Xie H., Liu Y., Gao P., Yang X., Shen Z. Effect of metformin on all-cause and cardiovascular mortality in patients with coronary artery diseases: a systematic review and an updated meta-analysis. Cardiovasc. Diabetol. 2019. 18(1). 96. doi: 10.1186/s12933-019-0900-7.
- Yang Q., Yuan H., Chen M., Qu J., Wang H., Yu B., Chen J. et al. Metformin ameliorates the progression of atherosclerosis via suppressing macrophage infiltration and inflammatory responses in rabbits. Life Sci. 2018. 198. 56-64. doi: 10.1016/j.lfs.2018.02.017.

/27.jpg)
/28.jpg)