Резюме
Мета: вивчити патофізіологічний взаємозв’язок вітаміну D та інтерлейкіну-6 (ІЛ-6), а також їх роль у розвитку анемії запалення в дітей раннього віку iз гострими запальними бактеріальними захворюваннями органів дихання. Матеріали та методи. Визначили вміст 25(OH)D3, ІЛ-6 та феритину в 40 дітей раннього віку (середній вік 1,6 ± 0,4 року) методом імуноферментного аналізу. Основну групу становили 20 дітей із гострими запальними бактеріальними захворюваннями респіраторного тракту, серед яких у 14 пацієнтів був діагностований гострий бактеріальний бронхіт, а у 6 дітей — пневмонія. Пацієнти основної групи були розділені на дві підгрупи: перша включала 10 дітей з анемією запалення, друга — 10 дітей з гострими бактеріальними захворюваннями респіраторного тракту без проявів анемії. Групу порівняння становили 10 дітей із залізодефіцитною анемією без проявів запальних захворювань органів дихання. Контрольна група представлена 10 умовно здоровими дітьми. Результати. У першій підгрупі дітей виявлено рівень 25(ОН)D3 на нижній межі норми (29,99 (28,1; 36,5) нг/мл), у другій підгрупі спостерігалася його недостатність (27,4 (26,1; 31,2) нг/мл). Рівень забезпеченості 25(ОН)D3 був нижчим в 1,3–1,6 раза порівняно з показниками пацієнтів контрольної групи (43,0 (38,2; 47,0) нг/мл) (p < 0,05). Рівень 25(ОН)D3 в сироватці крові дітей з групи порівняння (38,0 (34,0; 41,0) нг/мл) не відрізнявся від даних контрольної групи (p > 0,05), але щодо основної групи був вірогідно вищим (p < 0,05). Аналіз умісту ІЛ-6 в сироватці крові дітей показав, що в першій підгрупі його рівень перевищував показники контрольної групи (5,63 (4,52; 5,74) пг/мл) (p < 0,05), але був статистично нижчим за дані, отримані в другій підгрупі (6,63 (4,82; 8,93) пг/мл) (p < 0,05). У той же час ми не виявили статистично значущої різниці між показниками груп порівняння і контролю (p > 0,05). При рівні забезпеченості вітаміном D нижче 30 нг/мл спостерігалося порушення встановлених взаємозв’язків та погіршення депонування заліза, що, у свою чергу, формує умови для подальшої життєдіяльності патогена. Висновки. Перебіг гострих запальних бактеріальних захворювань органів дихання в дітей раннього віку характеризується зниженням рівня вітаміну D у сироватці крові, при якому вмiст прозапальних цитокінів негативно корелює з рівнем вітаміну D. Розвиток анемії запалення свідчить про певний баланс про- та протизапальних факторів у патогенезі гострих запальних бактеріальних захворювань органів дихання в дітей раннього віку.
Background. The purpose was to study the pathophysiological relationship of vitamin D and interleukin-6 (IL-6) and its role in the development of anemia of inflammation in young children with acute inflammatory bacterial diseases of the respiratory tract. Materials and methods. The content of 25(OH)D3, IL-6, and ferritin was analyzed in 40 young children (average age 1.6 ± 0.4 years) by enzyme immunoassay. The basic group consisted of 20 children with acute inflammatory bacterial diseases of the respiratory tract: 14 patients were diagnosed with acute bacterial bronchitis, and 6 children were diagnosed with pneumonia. Patients of the basic group were divided into two subgroups: the first group consisted of 10 children with anemia of inflammation, the second — 10 children with acute bacterial diseases of the respiratory tract without manifestations of anemia. The comparison group consisted of 10 children with iron deficiency anemia without manifestations of inflammatory respiratory diseases. Ten apparently healthy children represented the control group. Results. In the first subgroup of children, the level of 25(OH)D3 was borderline (29.99 (28.1; 36.5) ng/ml), in the second subgroup, its insufficiency was observed (27.4 (26.1; 31.2) ng/ml). The level of 25(OH)D3 was 1.3–1.6 times lower than the indicators of patients in the control group (43.0 (38.2; 47.0) ng/ml) (p < 0.05). The level of 25(OH)D3
in the blood serum of children from the comparison group (38.0 (34.0; 41.0) ng/ml) did not differ from the data in the control group (p > 0.05), but compared to the basic group, it was significantly higher (p < 0.05). The analysis of the IL-6 content in the blood serum of children showed that in the first subgroup its level exceeded the values of the control group (5.63 (4.52; 5.74) pg/ml) (p < 0.05) but was statistically lower than the indices obtained in the second subgroup (6.63 (4.82; 8.93) pg/ml) (p < 0.05). At the same time, we did not find a statistically significant difference between the indices of the comparison and control groups (p > 0.05). When the level of vitamin D supply is below 30 ng/ml, the established relationships are violated and the deposition of iron worsens, which in turn creates conditions for the further vital activity of the pathogen. Conclusions. The course of acute inflammatory bacterial diseases of the respiratory system in young children is characterized by a decrease in the level of vitamin D3 in the blood serum whereby the content of pro-inflammatory cytokines negatively correlates with the level of vitamin D. The development of anemia of inflammation indicates a certain balance of pro- and anti-inflammatory factors in the pathogenesis of acute inflammatory bacterial diseases of the respiratory tract in young children.
Introduction
Anemia of inflammation (AI) is a disease similar to iron deficiency anemia, which is characterized by a decrease in the level of iron in the blood serum, but it is distinct in a protective iron redistribution character [1]. Pro-inflammatory cytokines play the leading role in the pathogenesis of the AI, which implement the immune defense mechanism to reduce the availability of iron for extracellular pathogens since the presence of iron creates favorable conditions for their growth and reproduction. Pro- and anti-inflammatory cytokines interfere with the iron uptake by erythroid precursors. At the same time, it promotes iron storage in macrophages by stimulating the expression of ferritin [2]. Meanwhile, the literature data indicate that vitamin D promotes erythropoiesis by increasing the proliferation of erythroid precursors and reducing pro-inflammatory cytokines. Vitamin D can suppress hepcidin expression due to decreasing the level of pro-inflammatory cytokines through direct regulation of HAMP gene transcription. The decrease in pro-inflammatory cytokines and hepcidin can elevate the bioavailability of iron for erythropoiesis and hemoglobin synthesis by restoring iron recirculation, preventing iron sequestration in macrophages, and eliminating impaired iron absorption, thereby protecting against AI [3–5]. In addition to lowering pro-inflammatory cytokines, vitamin D supports erythropoiesis by increasing the proliferation of erythroid units (BFU-E) and synergizes with erythropoietin to further enhance the proliferation of erythroid progenitor cells [6, 7].
The purpose: to study the pathophysiological relationship of vitamin D and interleukin-6 (IL-6), and its role in the development of anemia of inflammation in young children with acute inflammatory bacterial diseases of the respiratory tract.
Materials and methods
The study included 40 children aged 1 month to 3 years (the average age of patients was 1.6 ± 0.4 years). The basic group consisted of 20 children with acute inflammatory bacterial diseases of the respiratory tract: 14 patients (70 %) were diagnosed with acute bronchitis, 6 (30 %) — with pneumonia. Taking into account the hematological picture, the basic group was divided into two subgroups. The first subgroup included 10 children with anemia of inflammation, which was determined 4–5 days after the onset of the disease. The second subgroup consisted of 10 children without anemia. Ten children with iron deficiency anemia without inflammatory manifestations represented the comparison group. The control group included 10 apparently healthy children. The observation groups were matched by the age and sex of the children. All patients who were enrolled in the study routinely received a vitamin D3 supplement according to clinical protocols.
The content of IL-6, 25(OH)D3, ferritin in blood serum was determined by enzyme immunoassay using commercial kits Human Interleukin 6 ELISA Kit (Elabscience Biotechnology Inc., USA), 25OH Vitamin D Total ELISA (DIAsource ImmunoAssays S.A., Belgium), Ferritin ELISA (ORGENTEC Diagnostika GmbH, Germany).
The normality test was carried out by the Shapiro-Wilk test. We used the method of correlation analysis with the calculation of the Spearman’s correlation coefficient. With uneven distribution of characters and a non-linear dependence, the median and quartiles were used (Me (Q25; Q75)). To assess the differences in indicators, the non-parametric Mann-Whitney U-test was calculated as a non-parametric analogue of the Student’s criterion. The differences were considered significant at p < 0.05.
All procedures performed in studies involving human participants were under the ethical standards of the institutional and national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants enrolled in the study. The full data set by children, their parents, and physician that support the findings of this study are not publicly available due to the restrictions of the ethics approval originally obtained.
Results and discussion
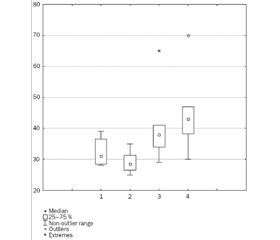
Based on the literature data indicating the important role of vitamin D and IL-6 in the pathogenesis of AI, we studied their content in children under observation. The results are shown in Fig. 1 and 2.
As can be seen from the data presented, the acute bacterial diseases of the respiratory system in young children occurred against the background of insufficient vitamin D supply, the content of which was 28.6 (26.6; 31.7) ng/ml. Taking into account the fact that the aforementioned vitamin is categorically necessary for the normal functioning of the immune system and given the anamnesis data, which indicated that all children included in the study were routinely administered prophylactic doses of the vitamin, a possible increased consumption of 25(OH)D3 is likely to result from an infectious process. When further analyzing the data obtained, we drew attention to the different degree of vitamin D provision in two comparison subgroups. So, in the first subgroup of children the level of 25(OH)D3 was borderline (29.99 (28.1; 36.5) ng/ml), while in the second subgroup, we registered its insufficiency (27.4 (26.1; 31.2) ng/ml). At the same time, the level of provision of 25(OH)D3 was 1.3–1.6 times lower than the indicators of patients in the control group (43.0 (38.2; 47.0) ng/ml) (p < 0.05).
/16.jpg)
Comparing the results obtained in the comparison group, it was noted that the level of 25(OH)D3 in blood serum (38.0 (34.0; 41.0) ng/ml) did not differ from the data in the control group (p > 0.05), but compared to the basic group it was significantly higher (p < 0.05).
Taking into account the numerous data on the role of IL-6 in the development of inflammation, we studied its content in the study groups.
The next stage of our work was to study the content of IL-6 in the blood serum, which has a pro-inflammatory orientation. As can be seen from the data in Fig. 2, the development of the inflammatory process in the respiratory tract was accompanied by an almost threefold increase in the content of IL-6 in the blood serum (6.13 (4.52; 8.93) pg/ml) (p < 0.05). The analysis of the IL-6 content in the blood serum of children from the observation groups, taking into account the characteristics of the subgroups, showed that in the first subgroup its level exceeded the indicators of the control group (5.63 (4.52; 5.74) pg/ml) (p < 0.05) but was statistically lower than the data obtained in the second subgroup (6.63 (4.82; 8.93) pg/ml) (p < 0.05).
At the same time, we did not find a statistically significant difference between the indices of the comparison and control groups (p > 0.05). Pro-inflammatory cytokines, among which IL-6 plays a central role, disrupt iron uptake by erythroid precursors and promote iron storage in macrophages by stimulating ferritin expression [2]. Our results are consistent with literature data, which established that IL-6 is a mediator responsible for increasing ferritin levels [9]. Decreased iron levels, accompanied by increased plasma ferritin and hepcidin levels, are characteristic of inflammatory anemia, while iron deficiency anemia is characterized by iron deficiency accompanied by low plasma ferritin and hepcidin levels [1]. Iron deficiency and increased production and activation of leukocytes realize a protective function due to the production of erythrocytes and an increase in their survival. Leukocytosis and a decrease in iron content reduce the number of erythroid precursors, and the activation of macrophages shortens the life span of erythrocytes. The long life expectancy of erythrocytes mitigates the effects of decreased erythropoiesis during most acute infections [1].
Zittermann A. et al. described the vitamin D-induced enhancement of the destruction of pathogens by mediators of antibacterial activity — neutrophils and α-defensins [10]. Probably, the level of vitamin D determined by us allows ensuring the implementation of its physiological functions in the body, however, presumably, its content is insufficient for the implementation of an immune response at the local level in the fight against pathogens. These results are consistent with the proposed mechanisms underlying the relationship between the concentration of vitamin D and the development of AI, the main pathogenetic link of which is the dysregulation of iron due to the sequestration of iron in the cells of the reticuloendothelial system as a result of the action of pro-inflammatory markers [8].
Previously, we studied the data concerning the level of ferritin in patients of the study groups, and observed its increase in the first subgroup by 1.6 times compared with the control group (p < 0.05) (56.51 (48.0; 63.0) and 29.0 (16.0; 50.0) ng/ml, respectively) and 1.5 times versus the comparison group (43.51 (23.0; 48.0) ng/ml) (p < 0.05) [11].
Taking into account the fact that AI is protective and based on the above literature data, we studied the relationship of vitamin D (depending on its level), interleukin-6, ferritin, and hemoglobin.
These data make it clear that when the level of vitamin D supply is below 30 ng/ml, the established relationships are violated and the deposition of iron worsens, which in turn creates conditions for the further vital activity of the pathogen. It should be noted that the studies by Bacchetta J. et al. demonstrated that in vivo vitamin D supplementation was predominantly accompanied by a decrease in serum ferritin levels [3]. The ultimate significance of this is not well understood but may reflect a generalized anti-inflammatory response due to an increased vitamin D concentration in the body. In turn, Bacchetta J. et al. showed that an increase in the concentration of vitamin D in the body affects the expression of hepcidin by reducing the circulation of the pro-inflammatory cytokine IL-6 [3].
Conclusions
1. The course of acute bacterial respiratory diseases in young children is characterized by a decrease in the level of vitamin D3 in the blood serum, in which the level of pro-inflammatory cytokines negatively correlates with the level of vitamin D.
2. The development of anemia of inflammation indicates a certain balance of pro- and anti-inflammatory factors in the pathogenesis of acute bacterial respiratory diseases in young children.
Conflicts of interests. Authors declare the absence of any conflicts of interests and their own financial interest that might be construed to influence the results or interpretation of their manuscript.
Received 08.01.2021
Revised 26.01.2021
Accepted 01.02.2021
Список литературы
1. Ganz T. Anemia of Inflammation. The New England Journal of Medicine. 2019. 381. 1148-1157. doi: 10.1056/nejmra1804281.
2. Arezes J., Nemeth E. Hepcidin and iron disorders: new biology and clinical approaches. International Journal of Laboratory Hematology. 2015. 37(1). 92-98. doi: 10.1111/ijlh.12358.
3. Bacchetta J., Zaritsky J.J., Sea J.L., Chun R.F., Lisse T.S. et al. Suppression of iron-regulatory hepcidin by vitamin D. Journal of the American Society of Nephrology. 2014. 25. 564-572. doi: 10.1681/asn.2013040355.
4. Zughaier S.M., Alvarez J.A., Sloan J.H., Konrad R.J., Tangpricha V. The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes. Journal of Clinical and Translational Endocrinology. 2014. 1. 19-25. doi: 10.1016/j.jcte.2014.01.003.
5. Nemeth E., Ganz T. Anemia of inflammation. Hematology/Oncology Clinics of North America. 2014. 28. 671-681. doi: 10.1371/journal.pone.0093283.
6. Alon D.B., Chaimovitz C., Dvilansky A. Novel role of 1,25(OH)2D3 in induction of erythroid progenitor cell proliferation. Experimental Hematology. 2002. 30. 403-409. doi: 10.1016/s0301-472x(02)00789-0.
7. Aucella F., Scalzulli R.P., Gatta G. Calcitriol increases burst-forming unit-erythroid proliferation in chronic renal failure. A synergistic effect with r-HuEpo. Nephron Clinical Practice. 2003. 95. 121-127. doi: 10.1159/000074837.
8. Weiss G., Ganz T., Goodnough L.T. Anemia of inflammation. Blood. 2019. 133(1). 40-50. doi: 10.1182/blood-2018-06-856500.
9. Teke H.U., Cansu D.U., Yildiz P., Temiz G., Bal C. Clinical significance of serum IL-6, TNF-a, Hepcidin, and EPO levels in anaemia of chronic disease and iron deficiency anaemia: The laboratory indicators for anaemia. Biomedical Research. 2017. 28(6). 2704-2710.
10. Zittermann A., Pilz S., Hoffmann H., Marz W. Vitamin D and airway infections: a European perspective. European Journal of Medical Research. 2016. 21. 14. doi: 10.1186/s40001-016-0208-y.
11. Lezhenko H.O., Pogribna A.O. The role of hepcidin in the pathogenetic mechanisms of anemia of inflammation development in young children with acute inflammatory bacterial diseases of the respiratory system. Zaporozhye Medical Journal. 2020. 22(4). 473-478. doi: 10.14739/2310-1210.2020.4.208356.
12. Wang F.D., Zhou D.B., Li S.L., Wang X., Zhang J.P., Duan M.H. Effect of recombinant human erythropoietin on hepcidin mRNA expression in patients with multiple myeloma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011. 19(2). 390-394.
/16.jpg)

/17.jpg)