Журнал «Здоровье ребенка» Том 16, №2, 2021
Вернуться к номеру
Ендоскопічно-морфологічна характеристика верхніх відділів шлунково-кишкового тракту в дітей із харчовою гіперчутливістю
Авторы: L.M. Bubyr, T.O. Kryuchko, I.M. Nesina, B.M. Filenko, T.O. Pedchenko
Ukrainian Medical Stomatological Academy, Poltava, Ukraine
Рубрики: Педиатрия/Неонатология
Разделы: Клинические исследования
Версия для печати
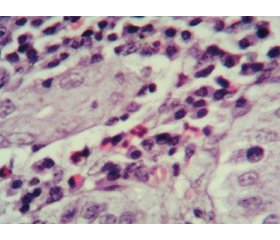
Актуальність. Останнім часом в педіатричній практиці все більше уваги приділяється вивченню алергічних уражень різних відділів шлунково-кишкового тракту, а ряд невирішених питань стосовно проблем своєчасної діагностики та лікування еозинофільних уражень органів травлення обумовлює актуальність цієї тематики. Мета: вивчити ендоскопічно-морфологічні зміни верхніх відділів шлунково-кишкового тракту в дітей із гастроінтестинальними симптомами харчової гіперчутливості. Матеріали та методи. У дослідженні взяли участь 34 дитини віком від 6 до 15 років із клінічними ознаками ураження верхніх відділів травного тракту на тлі реакцій харчової гіперчутливості. Залежно від рівня загального IgE учасники дослідження були розподілені на дві групи. Першу групу становили 18 дітей з IgE-незалежними алергічними реакціями на їжу (Me (Q1-Q3) 35,0 (28,0–77,5)). До другої групи було включено 16 пацієнтів з IgE-обумовленими алергічними проявами харчової гіперчутливості (Me (Q1-Q3) 240,5 (158,0–475,8)). Для досягнення поставленої мети всім дітям було проведено фіброезофагогастродуоденоскопію з подальшим забором матеріалу та наступною морфологічною оцінкою біоптатів. Результати. Згідно з результатами ендоскопічного обстеження пацієнтів, структура уражень верхніх відділів шлунково-кишкового тракту залежно від переважання IgE-незалежних чи IgE-опосередкованих гастроінтестинальних симптомів харчової гіперчутливості не мала статистично значущих відмінностей. Морфологічні характеристики мали певні відмінності в досліджуваних групах й характеризувалися превалюванням еозинофільної інфільтрації в дітей з IgE-обумовленими реакціями харчової гіперчутливості. Висновки. В ендоскопічній картині дітей із гастроінтестинальними проявами харчової алергії переважають ізольовані ураження шлунка. Морфологічні ж ознаки хронічного гастриту характеризуються змінами покривного епітелію за рахунок вираженої поліморфноклітинної запальної інфільтрації у власній пластинці слизової оболонки з переважанням лімфоцитів і плазмоцитів та порівняно меншою кількістю нейтрофілів, еозинофілів та макрофагів. При цьому активність еозинофільного запального процесу була більш вираженою в групі дітей з IgЕ-обумовленими реакціями харчової гіперчутливості (r = 0,652; р < 0,01).
Background. In pediatric practice, increasing attention has been recently paid to the study of allergic disorders in various parts of the gastrointestinal tract, and a number of unresolved issues regarding the timely diagnosis and treatment of eosinophilic lesions of the digestive system determines the relevance of this topic. The purpose of the research was to study the endoscopic and morphological changes of the upper gastrointestinal tract in children with gastrointestinal symptoms of food hypersensitivity. Materials and methods. The study enrolled 34 children aged from 6 to 15 years with clinical signs of disorders of the upper digestive tract against the background of food hypersensitivity reactions. Depending on the level of total IgE, study participants were divided into two groups. The first group consisted of 18 children with IgE-independent allergic reactions to food (Me (Q1-Q3) 35.0 (28.0–77.5)). The second group included 16 patients with IgE-induced allergic manifestations of food hypersensitivity (Me (Q1-Q3) 240.5 (158.0–475.8)). To achieve the aim of the research, all children underwent fibroesophagogastroduodenoscopy with subsequent sampling and morphological evaluation of biopsy specimen. Results. According to the results of endoscopic examination of patients, the structure of lesions of the upper gastrointestinal tract depending on the predominance of IgE-independent or IgE-mediated gastrointestinal symptoms of food hypersensitivity did not have statistically significant differences. Morphological characteristics had some differences in the study groups and were represented by the prevalence of eosinophilic infiltration in children with IgE-induced food hypersensitivity reactions. Conclusions. The isolated gastric lesions prevailed in the endoscopic presentation of children with gastrointestinal manifestations of food allergy, and morphological signs of chronic gastritis are characterized by changes in the surface epithelium due to pronounced polymorphonuclear inflammatory infiltration in the lamina propria with a predominance of lymphocytes, neutrophils and plasma cells. The degree of activity of the eosinophilic inflammatory process was more pronounced in the group of children with IgE-induced food hypersensitivity reactions (r = 0.652; p < 0.01).
харчова алергія; патологія шлунково-кишкового тракту; еозинофільний гастроентерит; діагностика; морфологічні ознаки
food allergy; pathology of the gastrointestinal tract; eosinophilic gastroenteritis; diagnosis; morphological signs
Background
Materials and methods
Results and discussion
Conclusions
- Уманец Т.Р., Шадрин О.Г., Клименко В.А. и др. Основные положения руководства по ведению больных с аллергией к коровьему молоку. Часть І. Эпидемиология и аллергены коровьего молока. Современная педиатрия. 2015. 1(65). 16-22.
- Охотникова Е.Н., Черныш Ю.Р. Гастроинтестинальная аллергия в практике педиатра и детского аллерголога. Здоровье ребeнка. 2015. 4. 15-21.
- Боброва В.І., Богданова Т.А. Гастроінтестинальна гіперреактивність та її значення у формуванні хронічного алергічного гастриту у дітей. Современная педиатрия. 2016. 1(73). 127-129. doi: 10.15574/SP.2016.73.127.
- Jensen E.T., Martin C.F., Kappelman M.D., Dellon E.S. Prevalence of eosinophilic gastritis, gastroenteritis, and colitis: estimates from a national administrative database. J. Pediatr. Gastroenterol. Nutr. 2016. 62(1). 36-42. doi: 10.1097/MPG.0000000000000865.
- Захарова И.Н., Бережная И.В. Пищевая аллергия у детей: с чем связан ее рост? Медицинский совет. 2018. 17. 156-162.
- Muraro A., Werfel T., Hoffmann-Sommergruber K., Roberts G., Beyer K., Bindslev-Jensen C. et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy. 2014 Aug. 69(8). 1008-25. doi: 10.1111/all.12429.
- Makarova S.G., Namazova-Baranova L.S., Vishneva E.А., Ereshko O.А., Gordeeva I.G. Gastrointestinal food allergy in children. Voprosy sovremennoi pediatrii — Current Pediatrics. 2017. 16(3). 202-212. doi: 10.15690/vsp.v16i3.1730.
- Зайцева Ю.Г., Халева Е.Г., Жданова М.В., Новик Г.А. Не-IgE-зависимая пищевая аллергия у детей. Лечащий врач. 2018. 4. 31-34.
- Betty van Esch. Cows milk allergy. Avoidance versus tolerance: new concepts for allergy management. Utrecht, the Netherlands. 2011. 1. 10.
- Sharon Chinthrajah R., Hernandes Joseph D., Boyd Scott D., Galli Stephen J., Nadeau Kari C. Molecular and cellular mechanisms of food allergy and food tolerance. J. Allergy Clin. Immunol. 2016 Apr. 137(4). 984-997.
- Lwin T., Melton S., Genta R. Eosinophilic gastritis: histopathological characterization and quantification of the normal gastric eosinophil content. Mod. Pathol. 2011. 24. 556-63.
- Sunkara T., Rawla P., Yarlagadda K.S., Gaduputi V. Eosinophilic gastroenteritis: diagnosis and clinical perspectives. Clin. Exp. Gastroenterol. 2019. 12. 239-53.
- Reed C., Woosley J.T., Dellon E.S. Clinical characteristics, treatment outcomes, and resource utilization in children and adults with eosinophilic gastroenteritis. Dig. Liver Dis. 2015. 47(3). 197-201.
- Черниш Ю.Р., Охотнікова О.М. Клінічні прояви гастроінтестинальної форми харчової алергії у дітей і підходи до її діагностики. Здоров’я дитини. 2017. 12(5). 611-622.
- Богданова Т.А., Березенко В.С., Пьянкова А.В., Гурьева О.В., Голованенко Г.Н. Особливості перебігу хронічного гастродуоденіту на тлі харчової гіперчутливості у підлітків. Современная педиатрия. 2018. 3(91). 54-58. doi: 10.15574/SP.2018.91.54.
- Шадрін О.Г., Задорожна Т.Д., Гайдучик Г.А., Арчакова Т.М., Місник В.П. Клініко-морфологічні особливості алергічного ентероколіту в дітей раннього віку. Патологія. 2019. 16(2). 238-244.
- Сорокман Т.В., Попелюк О.-М.В., Лозюк І.Я. Особливості перебігу поєднаної патології верхніх відділів шлунково-кишкового тракту та алергодерматозів у дітей. Здоров’я дитини. 2017. 12(3). 324-328.
- Боткина А.С. Пищевая аллергия у детей: современный взгляд на проблему. Лечащий врач. 2012. 6. 24-26.
- Абатуров А.Е., Кайдашев И.П., Никулина А.А. и др. Противовоспалительные эффекты пробиотической терапии хронических гастродуоденитов у детей. Здоровье ребенка. 2020. 15(5). 287-293.
- Pesek R.D., Reed C.C., Collins M.H., Muir A.B., Fulkerson P.C., Menard-Katcher C. et al. Association between endoscopic and histologic findings in a multicenter retrospective cohort of patients with non-esophageal eosinophilic gastrointestinal disorders. Dig. Dis. Sci. 2020. 65. 2024-2035.
- Fujiwara Y., Tanoue K., Higashimori A., Nishida Y., Maruyama M., Itani S. et al. Endoscopic findings of gastric lesions in patients with eosinophilic gastrointestinal disorders. Endosc. Int. Open. 2020. 8(12). 1817-1825.
- Ashitani K., Tsuzuki Y., Yamaoka M., Ohgo H., Ichimura T., Kusano T. et al. Endoscopic features and diagnostic procedures of eosinophilic gastroenteritis. Internal Medicine. 2019. 58(15). 2167-2171.
- Calvani M., Anania C., Cuomo B., D’Auria E., Decimo F., Indirli G.C. et al. Non-IgE- or mixed IgE/non-IgE-mediated gastrointestinal food allergies in the first years of life: old and new tools for diagnosis. Nutrients. 2021. 13(1). 226. doi: 10.3390/nu13010226.
- Kryuchko T.O., Bubyr L.M., Nesina I.M., Tkachenko O.Y., Izmailova O.V., Poda O.A. et al. Ways of optimizing the diagnostics of food allergies in children based on the clinical and immunological criteria. Wiad. Lek. 2020. 73(10). 2255-2260.

/58.jpg)
/59.jpg)
/60.jpg)