Introduction
Leukemia is the most prevalent childhood malignancy worldwide and accounts for 30 % of cancers in children aged 0–14 years [1, 2]. The incidence rate of pediatric leukemia in Europe was 4.7 new cases of leukemia per 100,000 children in 2012, with wide variations between countries; at rates ranging from 3.1 new cases per 100,000 children in Greece to 7 new cases per 100,000 children in Germany [3]. Acute lymphoblastic leukemia (ALL) accounts for 80 % of pediatric leukemia [1, 2]. Although the cause of ALL remains unclear, it is believed to be multifactorial. In addition to prenatal factors, immunological factors, exogenous and/or endogenous exposures, genetic susceptibility, biological heterogeneity, infections, seasonally changing environmental risk factors, and rural population mixing are suggested to play a role [4–6].
One of the proposed hypotheses of the etiology of ALL is Greaves’ delayed infection hypothesis [7, 8]. According to this hypothesis, childhood ALL develop in two critical steps, with the first step (first hit) occurring in utero, at which time a pre-leukemic clone is generated due to endogenous and developmental stress during fetal lymphopoiesis. In the absence of the second critical step (second hit), ALL does not occur. The second hit occurs in the postnatal period, depending upon the timing of exposure to common childhood infections. In the second hit, leukemia-associated genetic changes occur in a small percentage of children exposed to the first hit. Epidemiological evidence suggests that reduced microbial exposure of children living in a hygienic environment during their early years will lead to an abnormal immune response in the later years, resulting in a proliferation of leukemic cells [7, 8].
The Coronavirus Disease 2019 (COVID-19) pandemic can be viewed as a natural experimental opportunity to test and validate the delayed infection hypothesis [9]. According to data from Norway, there has been a reduction in the number of children diagnosed with ALL since the COVID-19 lockdown [9].
The first COVID-19 case in Turkey was detected on 10 March 2020, and strict measures were adopted nationwide to stop the spread of the infection. These included closing schools and children’s playgrounds by 16 March 2020. Curfews and intercity travel restrictions were also imposed, and quarantine measures were implemented. Classroom education, with COVID-19 prevention measures, resumed in primary and secondary schools on 12 October 2020.
The purpose of the study: to test the theory that isolation of children at home resulted in a reduction in the number of new childhood ALL cases in the Turkish population by avoiding the “second hit” in the development of leukemia.
Materials and methods
Study population
This retrospective study included pediatric ALL patients diagnosed between 1 January 2019 and 1 January 2021 at the University of Health Sciences, Bursa Yüksek Ihtisas Training and Research Hospital, Department of Pediatric Hematology. We extracted data on pediatric ALL cases from the hospital’s information system. Patient anonymity and confidentially were ensured.
The study periods were from 15 March 2020 to 31 December 2020 (COVID-19 lockdown period) and from 15 March 2019 to 31 December 2019 (control period). The number of new cases was defined as patients referred to our department for the diagnosis and treatment of ALL for the first time.
The institutional ethics and research committee approved the study protocol and waived informed consent due to its retrospective methodology.
Statistical analysis
The distribution of variables was examined using the Kolmogorov-Smirnov test. Nonparametric quantitative data are reported as median and interquartile range (IQR). The Mann-Whitney U test was used to compare quantitative data. A chi-square test of homogeneity was used to test the hypothesis of no difference in the number of ALL diagnoses by month. In all the tests, a value of p < 0.05 was considered statistically significant.
All calculations were carried out using the Statistical Package for the Social Sciences software (SPSS version 21, SPSS Inc., Chicago, IL, USA).
Results
This study consecutively included all children aged 1–18 years with a new diagnosis of acute leukemia. Acute leukemia was diagnosed in 73 children aged 2–18 years between January 2019 and December 2020. Of these, there were 58 ALL cases and 15 acute myeloid leukemia cases. Thirty (51.7 %) of the cases were males, and 28 (48.2 %) of the cases were females. The median age of the girls was 10 years (IQR: 10), and the median age of the boys was 9 years (IQR: 7).
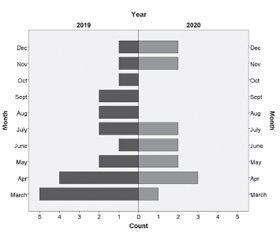
The number of ALL cases declined during the lockdown period, with 14 children diagnosed with ALL between 15 March 2020 and 31 December 2020 and 21 children diagnosed with ALL during the control period (33.3% reduction). As shown in fig. 1, no pediatric patients were diagnosed with ALL in August, September, and October 2020.
The distribution of children diagnosed with ALL according to age is shown in fig. 2. The median age of the patients diagnosed with ALL in control group (2019) and lockdown group was 10 years (IQR: 11 years) and 9 years (IQR: 6), respectively (p = 0.263). There were 12 (57 %) males in group 1 and 8 (57 %) males in group 2.
As shown by the chi-square test for homogeneity, there was no statistically significant difference in the distribution of the diagnoses according to specific months (p = 0.343).
Four of the 14 children diagnosed with ALL during the COVID-19 lockdown period had been diagnosed earlier with COVID-19 disease. In three of these four cases, the COVID-19 diagnosis was made 1 month before the ALL diagnosis. The diagnosis of COVID-19 was confirmed by a SARS-CoV-2 PCR test in only one of these four children [10]. In the patient with the positive PCR test for COVID-19, ALL was diagnosed 3 months after the COVID-19 diagnosis. Figure 3 shows the initial white bood cell, hemoglobin and platelet levels in children diagnosed with ALL.
Discussion
In our study, the number of newly diagnosed cases of ALL decreased in Turkey after 15 March 2020, when the country entered a lockdown period. Similarly, Jarvis et al. presented observational data indicating a significant decrease in the incidence of ALL in children during a COVID-19 outbreak [9].
In terms of ALL there are two opposite predictions based on Greaves’ etiological model [7, 8, 11]. The first prediction is that social isolation during the COVID-19 pandemic would reduce the number of newly diagnosed pediatric ALL cases. The second prediction is that widespreadstart of the COVID-19 pandemic [19]. exposure of children to severe acute respiratory syndrome (SARS)-CoV-2, even in asymptomatic children, may provide an important second hit and promote clonal evolution and clinical manifestations of ALL. Our findings were in line with the first prediction. Accordingly, during a SARS outbreak in Hong Kong in 2003, fewer cases of pediatric ALL were diagnosed in the country [12]. In addition, a significant, sharp decrease in many types of infectious diseases, such as chickenpox and measles, was reported during the SARS outbreak in 2013 [13]. Similarly, with the widespread closure of schools, businesses, places of worship, theatres and galleries, sports venues, and bars and restaurants to control the spread of COVID-19, there was a significant reduction in the number of emergency room visits and children hospitalized during winter period of 2020 in a study in USA linked to various respiratory viruses, including influenza and Respiratory Syncytial Virus [14].
In our study, one of the children newly diagnosed with ALL had been hospitalized with a diagnosis of COVID-19 3 months before an ALL diagnosis. Whether the COVID-19 diseaseacted as a second hit in this child is an important question. The second hit theory is supported by a reported increase in ALL cases in children in Milan approximately 2–3 months after the 2009/2010 H1N1 swine flu epidemic in Italy [15]. However, the role of subsequent responses to infectious agents in the pathogenesis of ALL is controversial, with some studies supporting such a role and others not supporting this idea [16]. The unique characteristics of the different populations studied and the retrospective nature of most reported studies may explain the ongoing controversy. Other indirect evidence for a role for the second hit in ALL is that protection against infections via vaccination appears to affect start of the COVID-19 pandemic [19] the development of childhood ALL. Studies conducted in the U.S. and in Finland reported that vaccination against Haemophilus influenzae type b reduced the risk of developing childhood ALL, especially in infancy [17].
Although there are a number of studies on the effects of the SARS-CoV-2 on patients with COVID-19, there is less known about indirect consequences for noninfected patients. We can assume that the COVID-19 pandemic led to a number of delayed ALL diagnosis [18–20]. Globally, routine pediatric visits were canceled, and emergency room visits decreased. There has also been a decline in the number of pediatric cancer cases diagnosed in Turkey since the start of the COVID-19 pandemic [19].
However, as the onset of ALL is acute in most cases, parents of children with symptoms would have been unlikely to delay attending the hospital. More in-depth analyses are needed to determine the impact of the pandemic on possible delays in pediatric cancer diagnoses.
The possibility that the reduction in patients diagnosed with ALL during the lockdown period was due to patients attending a center other than ours cannot be discounted. In addition, a much longer study period than that that in the current study is necessary, as bone marrow infiltration by blasts and associated pancytopenia are relatively slow (weeks to months) in ALL. Finally, the possibility that noninfectious environmental factors may trigger the onset of ALL in children with preexisting genetic susceptibility cannot be excluded [21, 22].
Conclusions
We observed a reduced incidence within the lockdown period, possibly related to the potential role of SARS-CoV-2 infection as a second hit in childhood ALL.
Large international data sets, including data on ALL diagnoses during lockdown periods and outside of lockdown periods, are needed to elucidate this issue.
Received 30.07.2021
Revised 05.09.2021
Accepted 16.09.2021
Список литературы
1. Jaime-Pérez J.C., Hernández-Coronado M., Hernández-De Los Santos J.A., Marfil-Rivera L.J., Gómez-Almaguer D. Monthly variation in diagnosis of acute lymphoblastic leukemia and survival outcome in children and adults: 15-year trends at a single center. Hematol. Transfus. Cell. Ther. 2021. 3. 2531-1379(20)31313-4.
2. Siegel D.A., Henley S.J., Li J., Pollack L.A., Van Dyne E.A., White A. Rates and trends of pediatric acute lymphoblastic leukemia — United States, 2001–2014. MMWR Morb. Mortal. Wkly. Rep. 2017. 66. 950-954.
3. OECD (2017), “Survival and mortality for leukaemia in children”, in Health at a Glance 2017: OECD Indicators, OECD Publishing, Paris. DOI: https://doi.org/10.1787/health_glance-2017-42-e.
4. Arellano-Galindo J., Barrera A.P., Jiménez-Hernández E., Zavala-Vega S., Campos-Valdéz G., Xicohtencatl-Cortes J., Ochoa S.A., Cruz-Córdova A., Crisóstomo-Vázquez M.D.P., Fernández-Macías J.C., Mejía-Aranguré J.M. Infectious agents in childhood leukemia. Arch. Med. Res. 2017. 48. 305-313.
5. Hein D., Borkhardt A., Fischer U. Insights into the prenatal origin of childhood acute lymphoblastic leukemia. Cancer Metastasis Rev. 2020. 39. 161-171.
6. Kinlen L.J. An examination, with a meta-analysis, of studies of childhood leukaemia in relation to population mixing. Br. J. Cancer. 2012. 107. 1163-1168.
7. Greaves M. The causation of childhood leukemia: a paradox of progress? Discov. Med. 2006. 31. 24-28.
8. Greaves M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat. Rev. Cancer. 2018. 18. 471-484.
9. Jarvis K.B., Lind A., LeBlanc M., Ruud E. Observed reduction in the diagnosis of acute lymphoblastic leukaemia in children during the COVID-19 pandemic. Acta Paediatr. 2021. 110. 596-597.
10. Asan A., Üstündağ Y., Koca N., Şimşek A., Sayan H.E., Parildar H., Dalyan Cilo B., Huysal K. Do initial hematologic indices predict the severity of COVID-19 patients? Turk. J. Med. Sci. 2021. 26. 51(1). 39-44.
11. Greaves M. COVID-19 and childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer. 2020. 67(12). e28481.
12. Li C.K., Zee B., Lee J., Chik K.W., Ha S.Y., Lee V. Impact of SARS on development of childhood acute lymphoblastic leukaemia. Leukemia. 2007. 21. 1353-1356.
13. Centre for Health Protection, Department of Health, The Government of Hong Kong SAR. www.chp.gov.hk/notifiable1518d.html.
14. Taub J.W., Ge Y., Xavier A.C. COVID-19 and childhood acute lymphoblastic leukemia. Pediatr. Blood Cancer. 2020. 67(7). e28400.
15. Cazzaniga G., Bisanti L., Randi G. et al. Possible role of pandemic AH1N1 swine flu virus in a childhood leukemia cluster. Leukemia. 2017. 31(8). 1819-1821.
16. Hwee J., Tait C., Sung L., Kwong J.C., Sutradhar R., Pole J.D. A systematic review and meta-analysis of the association between childhood infections and the risk of childhood acute lymphoblastic leukaemia. Br. J. Cancer. 2018 Jan. 118(1). 127-137.
17. Groves F., Auvinen A., Hakulinen T. Haemophilus influenzae type b vaccination and risk of childhood leukemia in a vaccine trial in Finland [Abstract]. Ann. Epidemiol. 2000. 10. 474.
18. Ding Y.Y., Ramakrishna S., Long A.H., Phillips C.A., Montiel-Esparza R., Diorio C.J., Bailey L.C. et al. Delayed cancer diagnoses and high mortality in children during the COVID-19 pandemic. Pediatr. Blood Cancer. 2020. 67. e28427.
19. Kutluk M.T., Ahmed F., Kirazlı M., Bajin İ.Y., Müngen E., Ekinci S., Yıldız F. The effect of the COVID-19 pandemic on paediatric cancer care: lessons learnt from a major paediatric oncology department in Turkey. Ecancermedicalscience. 2021. 15. 1172.
20. Kourti M., Markozannes G., Bouka P., Bouka E., Ntzani E., Petridou E.T. Pediatric cancer registration fluctuation in Greece due to COVID-19 pandemic and changes in health care delivery. Pediatr. Blood Cancer. 2021. 68(4). e28777.
21. Filippini T., Hatch E.E., Rothman K.J., Heck J.E., Park A.S., Crippa A., Orsini N., Vinceti M. Association between outdoor air pollution and childhood leukemia: A systematic review and dose-response meta-analysis. Environ. Health Perspect. 2019. 127(4). 46002.
22. Schüz J., Erdmann F. Environmental Exposure and Risk of Childhood Leukemia: An Overview. Arch. Med. Res. 2016 Nov. 47(8). 607-614.

/8.jpg)
/9.jpg)