Международный эндокринологический журнал Том 18, №1, 2022
Вернуться к номеру
Зв’язок між рівнями гормонів щитоподібної залози, інсулінорезистентністю та індексом маси тіла в пацієнтів із субклінічним гіпотиреозом
Авторы: Kasim Okan (1), Mehmet Sencan (2), Gulhan Duman (2)
(1) — Ege University, Faculty of Medicine, Izmir, Turkey
(2) — Sivas Cumhuriyet University, Faculty of Medicine, Sivas, Turkey
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
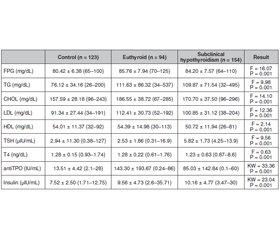
Актуальність і мета дослідження. Гіпотиреоз — поширене захворювання щитоподібної залози, із домінуванням серед жінок. У загальній популяції його поширеність становить 2–5 %, тоді як у жінок цей показник у 10 разів вищий, ніж у чоловіків. Інсулінорезистентність, одна з найбільш обговорюваних проблем, розглядається як неадекватна відповідь на дію інсуліну в периферичних тканинах, незважаючи на нормальну секреторну функцію клітин острівців підшлункової залози. У цьому дослідженні ми проаналізували взаємозв’язок між рівнями гормонів щитоподібної залози, індексом маси тіла та інсулінорезистентністю, розрахованою за допомогою гомеостатичної моделі оцінки інсулінорезистентності (HOMA-IR), кількісного індексу перевірки чутливості до інсуліну (QUICKI) та індексу атерогенності плазми в осіб із субклінічним гіпотиреозом на тлі замісної терапії левотироксином. Матеріали та методи. Клінічні та лабораторні дані приблизно 14 000 пацієнтів віком від 18 до 60 років були ретроспективно оцінені. Після застосування критеріїв виключення до дослідження було включено 371 відповідну особу. Усі особи розділені на три групи за рівнем тиреотропного гормону (ТТГ). Група 1 — це еутиреоїдні пацієнти із рівнем ТТГ 0,27–4,2 мкМО/мл, які отримують лікування левотироксином. Група 2 — пацієнти із субклінічним гіпотиреозом із рівнем ТТГ від 4,2 до 10 мкМО/мл. Група 3 — здорова контрольна група з рівнем ТТГ 0,27–4,2 мкМО/мл. Результати. Група пацієнтів у стані еутиреозу мала найвищий ІМТ (25,66 ± 3,36 кг/м2). З іншого боку, ІМТ був вищим у групі осіб із субклінічним гіпотиреозом (24,0400 ± 3,8436 кг/м2), ніж у контрольній групі (22,48 ± 2,74 кг/м2) (p < 0,05). Рівні глюкози плазми натще, тригліцеридів сироватки, ліпопротеїнів низької щільності (ЛПНЩ), антитіл до тиреоїдної пероксидази (АТ-ТПО) та інсуліну були значно вищими в групах пацієнтів у стані еутиреозу та з субклінічним гіпотиреозом (p < 0,05). Примітно, що рівні загального холестерину, ЛПНЩ і АТ-ТПО були вищими в групі пацієнтів із гіпотиреозом у стані компенсації (р < 0,05). З іншого боку, не було різниці між цими пацієнтами та особами із субклінічним гіпотиреозом. Висновки. У дослідженні встановлено вірогідно підвищений рівень резистентності до інсуліну та рівень холестерину в пацієнтів із субклінічним гіпотиреозом. Тому ми припустили, що субклінічний гіпотиреоз також є фактором ризику розвитку інсулінорезистентності, серцево-судинних захворювань та метаболічного синдрому. Тому показники ліпідограми та інсулінорезистентність слід враховувати при лікуванні осіб із субклінічним гіпотиреозом. Враховуючи встановлену в дослідженні вагому кореляцію між HOMA та QUICKI, ми пропонуємо комбіноване використання індексів HOMA та QUICKI в цих пацієнтів. Потрібні подальші широкомасштабні дослідження для оцінки взаємозв’язку HOMA, QUICKI та ІМТ у виявленні резистентності до інсуліну в пацієнтів із субклінічним гіпотиреозом.
Background. Hypothyroidism is a common thyroid disorder with female predominance. In general population its prevalance is 2–5 % while 10 times higher in female than in men. Insulin resistance, one of the most discussed issues recently, is an inadequate response to insulin in peripheral tissues despite the normal secretory function of pancreatic islet cells. In this study, we analyzed relationship between thyroid hormone levels, body mass index and insulin resistance calculated with Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), Quantitative Insulin Sensitivity Check Index (QUICKI) and Atherogenic Index of Plasma (AIP) in SCH and euthyroid patients under levothyroxine treatment. Materials and methods. The clinical and laboratory data of approximately 14 000 patients between the ages of 18–60 were retrospectively evaluated. After these exclusion criteria were applied, 371 eligible individuals were included in the study. All 371 individuals divided into three groups according to TSH levels. Group 1 is eutyhroid patients under levothyroxine treatment with TSH levels between 0.27–4.2 μIU/mL. Group 2 is subclinical hypothyroid patients with TSH levels between 4.2–10 μIU/mL. Group 3 is healthy control group with TSH levels between 0.27–4.2 μIU/mL. Results. The euthyroid patient group has the highest (25.66 ± 3.36 kg/m2) mean BMI. On the other hand the mean BMI was higher in SCH (24.0400 ± 3.8436 kg/m2) group than in control group (22.48 ± 2.74 kg/m2) (p < 0.05). Fasting plasma glucose (FPG), serum triglyserid, low density lipoprotein (LDL), anti-thyroid peroxidase (TPO) and insulin levels were significantly higher in euthyroid patient and SCH groups (p < 0.05). Notably, total cholesterol, LDL and TPO levels were higher in euthyroid patient group (p < 0.05). On the other hand, there were no difference between euthyroid patients and SCH group. Conclusions. This study found significantly elevated insulin resistance and cholesterol levels in SCH patients, so we hypothesized that SCH is also a risk factor for insulin resistance disorders such as cardiovascular diseases and metabolic syndrome. As a consequence, lipid metabolism defects and insulin resistance should be screened and treated in SCH patients. Thanks to the strong and significant correlation between HOMA and QUICKI in our study, we suggest the combined use of HOMA and QUICKI in these patients. Further and large-scale studies are needed to evaluate the relationship of HOMA, QUICKI, AIP, and BMI in detecting insulin resistance in SCH patients.
субклінічний гіпотиреоз; рівень гормонів щитоподібної залози; інсулінорезистентність; індекс маси тіла
subclinical hypothyroidism; thyroid hormone levels; insulin resistance; body mass index
Introduction
Materials and methods
Results
Discussion
Conclusion
- Parretti H., Okosieme O., Vanderpump M. Current recommendations in the management of hypothyroidism: developed from a statement by the British Thyroid Association Executive. Br. J. Gen. Pract. 2016 Oct. 66(651). 538-40. doi: 10.3399/bjgp16X687493. PMID: 27688516. PMCID: PMC5033303.
- Bemben D.А., Winn P., Hamm R.М., Morgan L., Davis A., Barton E. Thyroid disease in the elderly. Part 1. Prevalence of undiagnosed hypothyroidism. J. Fam. Pract. 1994 Jun. 38(6). 577-82. PMID: 8195731.
- Pearce S.Н., Brabant G., Duntas L.Н., Monzani F., Peeters R.Р., Razvi S., Wemeau J.L. 2013 ETA Guideline: Management of Subclinical Hypothyroidism. Eur. Thyroid J. 2013 Dec. 2(4). 215-28. doi: 10.1159/000356507. Epub 2013 Nov 27. PMID: 24783053. PMCID: PMC3923601.
- Moller D.Е., Flier J.S. Insulin resistance — mechanisms, syndromes, and implications. N. Engl. J. Med. 1991 Sep 26. 325(13). 938-48. doi: 10.1056/NEJM199109263251307.
- Archer A.Е., Von Schulze A.Т., Geiger P.C. Exercise, heat shock proteins and insulin resistance. Philos. Trans. R. Soc. B. 2018 Jan 19. 373(1738). 20160529. doi: 10.1098/rstb.2016.0529. PMID: 29203714. PMCID: PMC5717529
- Buchanan T.А., Watanabe R.М., Xiang A.H. Limitations in surrogate measures of insulin resistance. J. Clin. Endocrinol. Metab. 2010 Nov. 95(11). 4874-6. doi: 10.1210/jc.2010-2167. PMID: 21051585. PMCID: PMC2968734.
- Godini A., Ghasemi A., Zahediasl S. The Possible Mechanisms of the Impaired Insulin Secretion in Hypothyroid Rats. PLoS One. 2015 Jul 1. 10(7). e0131198. doi: 10.1371/journal.pone.0131198.
- Luna-Vazquez F., Cruz-Lumbreras R., Rodríguez-Castelán J. et al. Association between the serum concentration of triiodothyronine with components of metabolic syndrome, cardiovascular risk, and diet in euthyroid post-menopausal women without and with metabolic syndrome. Springerplus. 2014 May 24. 3. 266. doi: 10.1186/2193-1801-3-266.
- Ortega E., Koska J., Pannacciulli N., Bunt J.С., Krakoff J. Free triiodothyronine plasma concentrations are positively associated with insulin secretion in euthyroid individuals. Eur. J. Endocrinol. 2008 Feb. 158(2). 217-21. doi: 10.1530/EJE-07-0592. PMID: 18230829. PMCID: PMC2408760.
- Papadopoulou A.М., Bakogiannis N., Skrapari I., Moris D., Bakoyiannis C. Thyroid Dysfunction and Atherosclerosis: A Systematic Review. In Vivo. 2020 Nov-Dec. 34(6). 3127-3136. doi: 10.21873/invivo.12147.
- Chidakel A., Mentuccia D., Celi F.S. Peripheral metabolism of thyroid hormone and glucose homeostasis. Thyroid. 2005 Aug. 15(8). 899-903. doi: 10.1089/thy.2005.15.899. PMID: 16131332.
- Hsu W.Н., Tseng C.W., Huang Y.Т., Liang C.С., Lee M.Y., Chen S.C. Common Risk Factors in Relatives and Spouses of Patients with Type 2 Diabetes in Developing Prediabetes. Healthcare (Basel). 2021 Aug. 9(8). 1010. doi: 10.3390/healthcare9081010.
- Fernández-Macías J.С., Ochoa-Martínez A.С., Varela-Silva J.А., Pérez-Maldonado I.N. Atherogenic Index of Plasma: Novel Predictive Biomarker for Cardiovascular Illnesses. Arch. Med. Res. 2019 Jul. 50(5). 285-294. doi: 10.1016/j.arcmed.2019.08.009.
- Weetman A.Р., McGregor A.M. Autoimmune thyroid disease: further developments in our understanding. Endocr. Rev. 1994 Dec. 15(6). 788-830. doi: 10.1210/edrv-15-6-788.
- Owecki M., Nikisch E., Sowiński J. Hypothyroidism has no impact on insulin sensitivity assessed with HOMA-IR in totally thyroidectomized patients. Acta Clin. Belg. 2006 Mar-Apr. 61(2). 69-73. doi: 10.1179/acb.2006.013.
- Tuzcu A., Bahceci M., Gokalp D., Tuzun Y., Gunes K. Subclinical hypothyroidism may be associated with elevated high-sensitive c-reactive protein (low grade inflammation) and fasting hyperinsulinemia. Endocr. J. 2005 Feb. 52(1). 89-94. doi: 10.1507/endocrj.52.89.
- Sengupta S., Jaseem T., Ambalavanan J., Hegde A. Homeostatic Model Assessment-Insulin Resistance (HOMA-IR 2) in Mild Subclinical Hypothyroid Subjects. Indian J. Clin. Biochem. 2018 Apr. 33(2). 214-217. doi: 10.1007/s12291-017-0647-4.
- Al Sayed A., Al Ali N., Bo Abbas Y., Alfadhli E. Subclinical hypothyroidism is associated with early insulin resistance in Kuwaiti women. Endocr. J. 2006 Oct. 53(5). 653-7. doi: 10.1507/endocrj.k06-018.
- Aksoy N., Yeler M.Т., Ayan N.N., Ozkeskin A., Ozkan Z., Serin N.O. Association between thyroid hormone levels and insulin resistance and body mass index. Pak. J. Med. Sci. 2015 Nov-Dec. 31(6). 1417-20. doi: 10.12669/pjms.316.7560.
- Doğan H.О., Duman G.D. Assessment of the relationship between insulin resistance, atherogenic index of plasma and white blood cell count: A data mining study. Cumhur. Med. J. 2017. 39. 479-86. https://doi.org/10.7197/223.v39i29491.316368
- Shankar M., Narasimhappa S. Subclinical Hypothyroidism (SH) and Atherogenic Index of Plasma (AIP) in Women: A Case-Control Study From a Tertiary Care Hospital in South India. Cureus. 2020 Sep 24. 12(9). e10636. doi: 10.7759/cureus.10636. PMID: 33123449. PMCID: PMC7584321.
- Kazukauskiene N., Podlipskyte A., Varoneckas G., Mickuviene N. Insulin Resistance in Association with Thyroid Function, Psychoemotional State, and Cardiovascular Risk Factors. Int. J. Environ. Res. Public Health. 2021 Mar 25. 18(7). 3388. doi: 10.3390/ijerph18073388. PMID: 33805872. PMCID: PMC8036436.
- Mn S., Km S., Prashant A., Doddamani P., Sv S. Effect of insulin resistance in assessing the clinical outcome of clinical and subclinical hypothyroid patients. J. Clin. Diagn. Res. 2015 Feb. 9(2). OC01-4. doi: 10.7860/JCDR/2015/9754.5513. Epub 2015 Feb 1. PMID: 25859477. PMCID: PMC4378759.
- Liu J., Duan Y., Fu J., Wang G. Association Between Thyroid Hormones, Thyroid Antibodies, and Cardiometabolic Factors in Non-Obese Individuals With Normal Thyroid Function. Front. Endocrinol. (Lausanne). 2018 Apr 5. 9. 130. doi: 10.3389/fendo.2018.00130. PMID: 29674996. PMCID: PMC5895644.
- Blaslov K., Gajski D., Vucelić V., Gaćina P., Mirošević G., Marinković J., Vrkljan M., Rotim K. The association of subclinical insulin resistance with thyroid autoimmunity in euthyroid individuals. Acta Clin. Croat. 2020 Dec. 59(4). 696-702. doi: 10.20471/acc.2020.59.04.16. PMID: 34285440. PMCID: PMC8253084.
- Cengiz H., Demirci T., Varim C., Tamer A. The effect of Thyroid Autoimmunity on Dyslipidemia in patients with Euthyroid Hashimoto Thyroiditis. Pak. J. Med. Sci. 2021 Sep-Oct. 37(5). 1365-1370. doi: 10.12669/pjms.37.5.3883. PMID: 34475913. PMCID: PMC8377896.
- Brenta G., Berg G., Arias P., Zago V., Schnitman M., Muzzio M.L., Sinay I., Schreier L. Lipoprotein alterations, hepatic lipase activity, and insulin sensitivity in subclinical hypothyroidism: response to L-T(4) treatment. Thyroid. 2007 May. 17(5). 453-60. doi: 10.1089/thy.2006.0302.

/49.jpg)
/50.jpg)
/51.jpg)
/52.jpg)