Международный эндокринологический журнал Том 18, №5, 2022
Вернуться к номеру
Об’єм щитоподібної залози і частота тиреоїдних вузлів у пацієнтів з порушенням глікемії натще і цукровим діабетом 2-го типу
Авторы: V.I. Pankiv (1), T.Yu. Yuzvenko (1), I.V. Pankiv (2)
(1) — Ukrainian Scientific and Practical Center of Endocrine Surgery, Transplantation of Endocrine Organs and Tissues of the Ministry of Health of Ukraine, Kyiv, Ukraine
(2) — Bukovinian State Medical University, Chernivtsi, Ukraine
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
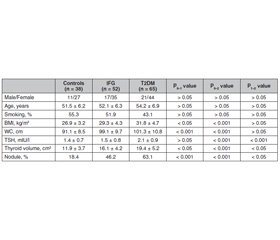
Актуальність. Взаємозв’язок між інсулінорезистентністю й наявністю вузлів щитоподібної залози не з’ясований. Інсулінорезистентність асоціюється зі збільшенням об’єму щитоподібної залози й поширеністю тиреоїдних вузлів у пацієнтів з метаболічним синдромом. Зазвичай метформін у пацієнтів із цукровим діабетом 2-го типу (ЦД2) може впливати на функцію і морфологію щитоподібної залози. Дані про взаємозв’язок морфологічних змін щитоподібної залози з порушенням вуглеводного обміну обмежені. Мета: проспективне дослідження було проведено для оцінки порушення глікемії натще (ПГН) і ЦД2 як факторів ризику збільшення об’єму щитоподібної залози й поширеності вузлів у йододефіцитному регуоні. Матеріали та методи. Проведено проспективне дослідження типу «випадок — контроль» у пацієнтів із ПГН і ЦД2. Дані були зібрані щодо всіх пацієнтів з нещодавно діагностованими ПГН і ЦД2 упродовж періоду із січня 2018 року до грудня 2020 року. 65 пацієнтів із ПГН і 52 пацієнти з ЦД2 були рандомізовані за віком, статтю і статусом куріння з 38 суб’єктами з нормальним вуглеводним метаболізмом. Усім учасникам проводили визначення тиреотропного гормону (ТТГ) і ультразвукове дослідження щитоподібної залози. Результати. Середній рівень ТТГ у групі ЦД2 (2,1 ± 0,9 мМО/л) був вірогідно вищим, ніж у контрольній групі (1,4 ± 0,7 мМО/л) і групі з ПГН (1,5 ± 0,8 мМО/л) (P < 0,001 для обох). Рівні автоантитіл до щитоподібної залози перебували в межах референсних значень й істотно не відрізнялися між трьома групами. Середній об’єм щитоподібної залози був вірогідно вищим у групах з ПГН (16,1 ± 4,2 см3) і ЦД2 (19,4 ± 5,2 см3), ніж у контрольній групі (11,9 ± 3,7 см3). У групі ЦД2 спостерігалася позитивна кореляція між ТТГ та ІМТ (r = 0,43; p < 0,01), а також між ТТГ і окружністю талії (r = 0,37; p < 0,01). Відсоток пацієнтів із вузлами щитоподібної залози також був вищим у групах з ПГН (46,2 %) і ЦД2 (63,1%), ніж у контрольній групі (18,4 %). Висновки. У пацієнтів з порушенням глікемії натще і цукровим діабетом 2-го типу вірогідно збільшені об’єм щитоподібної залози й поширеність тиреоїдних вузлів порівняно з контрольною групою.
Background. The relationship between insulin resistance and thyroid nodules is not clearly understood. Insulin resistance is associated with increased thyroid volume and nodule prevalence in patients with metabolic syndrome. Metformin commonly used in patients with type 2 diabetes mellitus (T2DM) might possibly alter thyroid function and morphology. Data on the association of thyroid morphology and abnormal glucose metabolism are limited. This prospective study was carried out to evaluate impaired fasting glucose (IFG) and T2DM as a risk factor for increased thyroid volume and nodule prevalence in iodine-deficient area. Materials and methods. This was a prospective case-control study in patients with IFG and T2DM. Data were gathered on all patients newly diagnosed with IFG and T2DM between January 2018 and December 2020. Sixty-five patients with IFG and 52 people with T2DM were randomly matched for age, gender, and smoking habits with 38 subjects with normal glucose metabolism. Serum thyroid-stimulating hormone (TSH) was evaluated, and thyroid ultrasonography was performed in all participants. Results. Mean TSH level in the T2DM group (2.1 ± 0.9 mIU/l) was significantly higher than in controls (1.4 ± 0.7 mIU/l) and in the IFG group (1.5 ± 0.8 mIU/l) (p < 0.001 for both). Thyroid autoantibody levels were within normal limits and did not differ significantly between 3 groups. Mean thyroid volume was significantly higher in the IFG (16.1 ± 4.2 cm3) and T2DM groups (19.4 ± 5.2 cm3) compared to controls (11.9 ± 3.7 cm3). In the T2DM group, there was a positive correlation between TSH and body mass index (r = 0.43; p < 0.01), and between TSH and waist circumference (r = 0.37; p < 0.01). The number of patients with thyroid nodules was also higher in the IFG (46.2 %) and T2DM groups (63.1 %) than in controls (18.4 %). Conclusions. The results suggest that patients with impaired glucose metabolism and type 2 diabetes mellitus have significantly increased thyroid volume and nodule prevalence.
цукровий діабет 2-го типу; порушення глікемії натще; вузол щитоподібної залози; об’єм щитоподібної залози
type 2 diabetes mellitus; impaired fasting glucose; thyroid nodule; thyroid volume
Introduction
Materials and methods
Results
Discussion
Conclusions
- Grani G., Sponziello M., Pecce V., Ramundo V., Durante C. Contemporary Thyroid Nodule Evaluation and Management. J. Clin. Endocrinol. Metab. 2020. 105(9). 2869-83. doi: 10.1210/clinem/dgaa322.
- Demiral Sezer S., Erdoğan Yücel E. Does insulin resistance trigger thyroid nodule? Intern. Emerg. Med. 2021. 16(8). 2105-2108. doi: 10.1007/s11739-021-02713-z.
- Tang Y., Yan T., Wang G., Chen Y., Zhu Y., Jiang Z. et al. Correlation between Insulin Resistance and Thyroid Nodule in Type 2 Diabetes Mellitus. Int. J. Endocrinol. 2017. 2017. 1617458. doi: 10.1155/2017/1617458.
- Aydoğan Y., Altay M., Ünsal O., Kaplanoğlu V., Çağır Y., Yıldız C. et al. An assessment of the relationship between thyroid nodule characteristics, insulin resistance and arterial stiffness in euthyroid nodular goiter. Endocrine. 2018. 62(2). 440-447. doi: 10.1007/s12020-018-1701-6.
- Kalra S., Aggarwal S., Khandelwal D. Thyroid Dysfunction and Type 2 Diabetes Mellitus: Screening Strategies and Implications for Management. Diabetes Ther. 2019. 10(6). 2035-2044. doi: 10.1007/s13300-019-00700-4.
- Haroon S.M., Khan K., Maqsood M., Iqbal S., Aleem M., Khan T.U. Exploring the Effect of Metformin to Lower Thyroid-Stimulating Hormone in Euthyroid and Hypothyroid Type-2 Diabetic Patients. Cureus. 2021. 13(2). e13283. doi: 10.7759/cureus.13283.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020. 43 (Suppl. 1). S14-S31. doi: 10.2337/dc20-S002. PMID: 31862745.
- Pasyechko N., Chukur O., Krytskyy T., Bob A. Iodine status of children and women of reproductive age in the Western region of Ukraine. International Journal of Endocrinology. 2019. 15(7). 541-547. https://doi.org/10.22141/2224-0721.15.7.2019.186057.
- Pankiv V.I., Yuzvenko T.Y., Pankiv I.V. Type 2 diabetes mellitus and subclinical hypothyroidism: focusing on the role of cholecalciferol. Problems of Endocrine Pathology. 2019. 68(2). 46-51. https://doi.org/10.21856/j-PEP.2019.2.07
- Mehalingam V., Sahoo J., Bobby Z., Vinod K.V. Thyroid dysfunction in patients with type 2 diabetes mellitus and its association with diabetic complications. J. Family Med. Prim. Care. 2020. 9(8). 4277-4281. doi: 10.4103/jfmpc.jfmpc_838_20.
- Wondmkun Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020. 13. 3611-3616. doi: 10.2147/DMSO.S275898.
- Kazukauskiene N., Podlipskyte A., Varoneckas G., Mickuviene N. Insulin Resistance in Association with Thyroid Function, Psychoemotional State, and Cardiovascular Risk Factors. Int. J. Environ. Res Public Health. 2021. 18(7). 3388. doi: 10.3390/ijerph18073388.
- Spira D., Buchmann N., Dörr M., Markus M.R.P., Nauck M., Schipf S. et al. Association of thyroid function with insulin resistance: data from two population-based studies. Eur. Thyroid J. 2022. 11(2). e210063. doi: 10.1530/ETJ-21-0063.
- Junik R., Kozinski M., Debska-Kozinska K. Thyroid ultrasound in diabetic patients without overt thyroid disease. Acta Radiol. 2006. 47(7). 687-91. doi: 10.1080/02841850600806308.
- Ortiga-Carvalho T.M., Chiamolera M.I., Pazos-Moura C.C., Wondisford F.E. Hypothalamus-Pituitary-Thyroid Axis. Compr. Physiol. 2016. 6(3). 1387-428. doi: 10.1002/cphy.c150027. PMID: 27347897.
- Rogers R.C., McDougal D.H., Hermann G.E. Leptin amplifies the action of thyrotropin-releasing hormone in the solitary nucleus: an in vitro calcium imaging study. Brain Res. 2011. 1385. 47-55. doi: 10.1016/j.brainres.2011.02.029.
- Onyemelukwe O.U., Ogoina D., Onyemelukwe G.C. Leptin concentrations in type 2 diabetes and non-diabetes Nigerian-Africans. Am. J. Cardiovasc. Dis. 2020. 10(4). 444-454. PMID: 33224595; PMCID: PMC7675163.
- Yu H., Li Q., Zhang M., Liu F., Pan J., Tu Y., Lu J. et al. Decreased Leptin Is Associated with Alterations in Thyroid-Stimulating Hormone Levels after Roux-en-Y Gastric Bypass Surgery in Obese Euthyroid Patients with Type 2 Diabetes. Obes Facts. 2019. 12(3). 272-280. doi: 10.1159/000499385.
- Chang Y.C., Hua S.C., Chang C.H., Kao W.Y., Lee H.L., Chuang LM. et al. High TSH Level within Normal Range Is Associated with Obesity, Dyslipidemia, Hypertension, Inflammation, Hypercoagulability, and the Metabolic Syndrome: A Novel Cardiometabolic Marker. J. Clin. Med. 2019. 8(6). 817. doi: 10.3390/jcm8060817.
- Matti B., Cohen-Hallaleh R. Overview of the 2015 American Thyroid Association guidelines for managing thyroid nodules and differentiated thyroid cancer. N. Z. Med. J. 2016. 129(1441). 78-86. PMID: 27607088.

/35.jpg)
/36.jpg)