Международный эндокринологический журнал Том 18, №5, 2022
Вернуться к номеру
Валідність і надійність шкали оцінки ефективності лікування цукрового діабету в Туреччині
Авторы: Esin Kavuran (1), Nihan Türkoğlu (2)
(1) — Department of Nursing Fundamentals, Nursing Faculty, Ataturk University, Erzurum, Turkey Central Campus-Yakutiye-Erzurum, Turkey
(2) — Department of Public Health Nursing, Nursing Faculty, Ataturk University, Erzurum, Turkey
Рубрики: Эндокринология
Разделы: Клинические исследования
Версия для печати
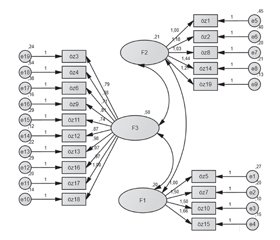
Актуальність. Проблема адаптації до лікування цукрового діабету (ЦД) перешкоджає досягненню компенсації, призводить до прогресування захворювання, а також до зростання витрат через хворобу. Аналіз ефективності лікування ЦД 2-го типу в Туреччині вказує на високу частку осіб з низьким рівнем комплаєнсу і компенсації ЦД. У дослідженні 1456 осіб із ЦД, які отримували інсулінотерапію, 29,7 % хворих повідомили, що вони не дотримуються призначеного режиму лікування. Мета: дослідження розроблено й проведено з метою вивчення валідності й надійності шкали оцінки ефективності лікування діабету (DMSS). Матеріали та методи. У дослідженні взяли участь 197 осіб, які перебували під спостереженням в амбулаторії університетської лікарні в період із травня по червень 2019 року. У них був діагностований ЦД принаймні один рік тому, і хворі погодилися брати участь у дослідженні. Для перевірки надійності (внутрішньої узгодженості) шкали оцінки ефективності медикаментозного лікування ЦД використовувався метод альфа Кронбаха. Результати. У результаті аналізу було встановлено, що шкала складається з 19 пунктів і трьох підпунктів. Значення альфа Кронбаха для всієї шкали та її підшкал становили 0,94; 0,89; 0,85 і 0,93 відповідно. Для перевірки 3-факторної структури шкали було проведено підтверджуючий факторний аналіз. Згідно з результатами підтверджуючого факторного аналізу, χ2/SD = 3,22 виявився меншим за прийнятне контрольне значення 5. Цей результат показує, що дані сумісні з моделлю. Крім того, інші результати були такими: RMSEA = 0,070; PNFI = 0,82; CFI = 0,99; RMR = 0,27; GFI = 0,94; AGFI = 0,88 і PGFI = 0,61. Висновки. Валідну, перевірену й стандартизовану 19-пунктову 5-бальну шкалу типу Лайкерта можна використовувати для визначення оцінки ефективності лікування хворих на цукровий діабет у популяції.
Background. As the problem of adaptation to diabetes management in chronic diseases prevents the success of treatment, it causes the disease process to progress negatively, other diseases and deaths to develop and the costs of the disease to increase. Type 2 diabetes mellitus treatment in Turkey concerns the rate of individuals with low compliance and continuity of the disease. In a study of 1,456 individuals with diabetes treated with insulin throughout Turkey, 29.7 % of patients reported that they did not adhere to the prescribed drug regimen. This study was designed to investigate the validity and reliability of the Diabetes Medication Self-Efficacy Scale. Materials and methods. The study was constituted with 197 individuals, who admitted to endocrinology outpatient clinic of a University Hospital between May and June 2019, and had diabetes diagnosis for at least one year and agreed to participate in the research. The descriptive and confirmatory factor analysis and Cronbach’s alpha internal consistency analysis were used during the Turkish adaptation phase of the Diabetes Medication Self-Efficacy Scale. Results. As a result of the analysis, the scale was determined to be consisted of 19 items and three sub-dimensions and explained 68.472 % of the total variance. Cronbach’s alpha values for the whole scale and its sub-scales were 0.94, 0.89, 0.85, and 0.93, respectively. Confirmatory factor analysis was performed to verify the 3-factor structure of the scale. According to the confirmatory factor analysis results, χ2/SD = 3.22 was found to be less than the acceptable reference value of 5. This finding shows that the data are compatible with the model. In addition, the other results were as follows: RMSEA = 0.070, PNFI = 0.82, CFI = 0.99, RMR = 0.27, GFI = 0.94, AGFI = 0.88 and PGFI = 0.61. When we look at these values, they are appeared to be in good alignment. Conclusions. This reliable, validated and standardized 19-item 5-point Likert type scale can be used to determine the medication self-efficacy for patients with diabetes in society.
цукровий діабет; медикаментозне лікування; ефективність; шкала оцінки
diabetes mellitus; medication; self-efficacy; validity; reliability
Introduction
Materials and methods
Results
/48_2.jpg)
Discussion
Conclusions
- Sun H., Saeedi P., Karuranga S., Pinkepank M., Ogurtsova K., Duncan B.B., Stein C. et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin. Pract. 2022. 183. 109119. doi: 10.1016/j.diabres.2021.109119.
- Khan M.A.B., Hashim M.J., King J.K., Govender R.D., Mustafa H., Al Kaabi J. Epidemiology of Type 2 Diabetes Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health. 2020. 10(1). 107-111. doi: 10.2991/jegh.k.191028.001.
- Jiang C., Wang Q. Analysis of Influencing Factors for Chronic Diseases: A Large Sample Epidemiological Survey from Liaoyang. Comput. Math. Methods Med. 2022. 2022. 1537906. doi: 10.1155/2022/1537906.
- Deters M.A., Laven A., Castejon A., Doucette W.R., Ev L.S., Krass I., Mehuys E. et al. Effective Interventions for Diabetes Patients by Community Pharmacists: A Meta-analysis of Pharmaceutical Care Components. Ann. Pharmacother. 2018. 52(2). 198-211. doi: 10.1177/1060028017733272.
- American Diabetes Association Professional Practice Committee, Draznin B., Aroda V.R., Bakris G., Benson G., Brown F.M., Freeman R., Green J. et al. 6. Glycemic Targets: Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022. 45 (Suppl. 1). S83-S96. doi: 10.2337/dc22-S006.
- Wormall S., Abbot S. Primary care clinical pharmacists and chronic disease medication adherence. Br. J. Gen. Pract. 2021. 71(712). 496. doi: 10.3399/bjgp21X717485.
- Blaslov K., Naranđa F.S., Kruljac I., Renar I.P. Treatment approach to type 2 diabetes: Past, present and future. World J. Diabetes. 2018. 9(12). 209-219. doi: 10.4239/wjd.v9.i12.209.
- Kumar A., Hejmady D.I., Thapar R., Kumar N., Holla R., Shetty V et al. Are Our Diabetic Patients Adherent to the Treatment? Curr. Diabetes Rev. 2021. 17(8). e022221191731. doi: 10.2174/1573399817666210223114010. P
- Krass I., Schieback P., Dhippayom T. Systematic Review or Metaanalysis Adherence to diabetes medication: a systematic review. Diabetic Medicine. 2015. 32. 725-37. DOI: 10.1111/dme.12651.
- Yavuz D.G., Ozcan S., Deyneli O. Adherence to insulin treatment in insulin-naïve type 2 diabetic patients initiated on different insulin regimens. Patient. Prefer. Adherence. 2015. 9. 1225-31. doi: 10.2147/PPA.S87935.
- Yavuz D.G., Bilen H., Sancak S., Garip T., Hekimsoy Z., Sahin I. et al. Impact of telephonic interviews on persistence and daily adherence to insulin treatment in insulin-naïve type 2 diabetes patients: dropout study. Patient Prefer. Adherence. 2016. 10. 851-61. doi: 10.2147/PPA.S100626.
- Peyrot M., Barnett A.H., Meneghini L.F., Schumm-Draeger P.M. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet. Med. 2012. 29(5). 682-9. doi: 10.1111/j.1464-5491.2012.03605.x.
- Erkuş A. Mismatch between the meanings of turkish equivalents of psychometric terms and the processes performed. Journal of Measurement and Evaluation in Education and Psychology. 2010. 1(2). 72-7.
- Sleath B., Carpenter D.M., Blalock S.J., Davis S.A., Hickson R.P., Lee C., Ferreri S.P. et al. Development of a new diabetes medication self-efficacy scale and its association with both reported problems in using diabetes medications and self-reported adherence. Patient Prefer. Adherence. 2016. 10. 1003-10. doi: 10.2147/PPA.S101349.
- Erdim L., Ergün A., Kuğuoğlu S. Reliability and validity of the Turkish version of the Physical Activity Questionnaire for Older Children (PAQ-C). Turk. J. Med. Sci. 2019. 49(1). 162-169. doi: 10.3906/sag-1806-212.
- Altan L., Çeliker R., Ercan İ., Birtane M., Akgün K., Zateri C. et al. The reliability and validity of the Turkish version of the Fibromyalgia Participation Questionnaire. Eur. J. Rheumatol. 2018. 5(1). 40-44. doi: 10.5152/eurjrheum.2017.16090.
- Sangwan A., Sangwan A., Punia P. Development and Validation of an Attitude Scale towards Online Teaching and Learning for Higher Education Teachers. TechTrends. 2021. 65(2). 187-195. doi: 10.1007/s11528-020-00561-w.
- Erefe İ. (Ed.) Research principles, processes and methods in nursing. İstanbul: Odak Offset; 2002. 169-88.
- Yurdugül H., Bayrak F. Valitidy validation measures in scale development studies: Comparison of scope validity index and Kappa statistics. Hacettepe University Faculty of Education Journal. 2012. 2. 264-71.
- Otieno-Odawa C.F., Kaseje D.O. Validity and reliability of data collected by community health workers in rural and peri-urban contexts in Kenya. BMC Health Serv. Res. 2014. 14 (Suppl 1). S5. doi: 10.1186/1472-6963-14-S1-S5.
- Tolchard B. Reliability and Validity of the Work and Social Adjustment Scale in Treatment-Seeking Problem Gamblers. J. Addict. Nurs. 2016. 27(4). 229-233. doi: 10.1097/JAN.0000000000000141.
- Tavşancil E. Measurement of attitudes and data analysis with SPSS. Ankara: Nobel Publishing; 2002.
- Guetterman T.C. Basics of statistics for primary care research. Fam. Med. Community Health. 2019. 7(2). e000067. doi: 10.1136/fmch-2018-000067.
- Cartagena-Ramos D., Fuentealba-Torres M., Rebustini F., Leite A.C.A.B., Alvarenga W.A., Arcêncio R.A. et al. Systematic review of the psychometric properties of instruments to measure sexual desire. BMC Med. Res Methodol. 2018. 18(1). 109. doi: 10.1186/s12874-018-0570-2.
- Zheng J., Wang Y., Ye X., Xiao L., Ye J., Li X., Zhong M. Validation of diabetes medication self-efficacy scale in Chinese with type 2 diabetes. Patient Prefer. Adherence. 2018. 12. 2517-2525. doi: 10.2147/PPA.S170144.
- Ali Z., Bhaskar S.B. Basic statistical tools in research and data analysis. Indian J. Anaesth. 2016. 60(9). 662-669. doi: 10.4103/0019-5049.190623.
- Erkorkmaz Ü., Etikan D., Demir O., Özdamar K. Sanisoğlu SY. Confirmatory factor analysis and fit indices: review. Turkiye Klinikleri J. Med. Sci. 2013. 33(1). 210-23. DOI:10.5336/medsci.2011-26747.
- Bajpai S.R., Bajpai R.C. Goodness of measurement: reliability and validity. International Journal of Medical Science and Public Health. 2014. 3(2). 112-5. DOI: 10.5455/ijmsph.2013.191120133.
- Stein C.M., Morris N.J., Hall N.B., Nock N.L. Structural Equation Modeling. Methods Mol. Biol. 2017. 1666. 557-580. doi: 10.1007/978-1-4939-7274-6_28.
- Atkinson T.M., Rosenfeld B.D., Sit L., Mendoza T.R., Fruscione M., Lavene D. et al. Using confirmatory factor analysis to evaluate construct validity of the Brief Pain Inventory (BPI). J. Pain Symptom Manage. 2011. 41(3). 558-65. doi: 10.1016/j.jpainsymman.2010.05.008.
- Yatim M.H., Wong Y.Y., Neoh C.F., Lim S.H., Hassali M.A., Hong Y.H. Factors influencing patients’ hypertension self-management and sustainable self-care practices: a qualitative study. Public Health. 2019. 173. 5-8. doi: 10.1016/j.puhe.2019.04.020.
- Messina R., Rucci P., Sturt J., Mancini T., Fantini M.P. Assessing self-efficacy in type 2 diabetes management: validation of the Italian version of the Diabetes Management Self-Efficacy Scale (IT-DMSES). Health Qual Life Outcomes. 2018. 16(1). 71. doi: 10.1186/s12955-018-0901-3.
- Bohanny W., Wu S.F., Liu C.Y., Yeh S.H., Tsay S.L., Wang T.J. Health literacy, self-efficacy, and self-care behaviors in patients with type 2 diabetes mellitus. J. Am. Assoc. Nurse Pract. 2013. 25(9). 495-502. doi: 10.1111/1745-7599.12017. Epub 2013 Mar 4. PMID: 24170654.
- Erol O., Enc N. Hypoglycemia Fear and Self-efficacy of Turkish Patients Receiving Insulin Therapy. Asian Nurs. Res (Korean Soc. Nurs. Sci). 2011. 5(4). 222-8. doi: 10.1016/j.anr.2011.12.001.

/48.jpg)
/49.jpg)
/50.jpg)
/51.jpg)