Международный неврологический журнал 1 (71) 2015
Вернуться к номеру
Epilepsy and Migraine: Neuroimaging and Neuropathophysiological Parallels
Авторы: Yevstigneiev V.V., Kistsen O.V., Sadokha K.A. - Belarusian Medical Academy of Postgraduate Education, Minsk; Sakovich R.A. - Minsk City Clinical Hospital, Minsk, Belarus
Рубрики: Неврология
Разделы: Клинические исследования
Версия для печати
Objective. The aim of our study was the comparison of the microstructure and neurometabolic disorders of the brain, as well as neuropathophysiological features of patients suffering from epilepsy and migraine. Methods. We investigated 60 patients suffering from migraine, aged 16–42 years (mean age was 29.30 ± 0.52 years) and 60 — with epilepsy, aged 18–51 years (mean age was 28.20 ± 0.98 years). 60 patients with migraine and 60 with epilepsy underwent diffusion tensor magnetic resonance imaging. 18 migraine and 28 epilepsy patients underwent proton nuclear magnetic resonance spectroscopy. All patients were examined using the computer electroencephalography. Migraine and epilepsy were diagnosed according to international guidelines. Results. The obtained data indicate not only a neuron tissue lesion in patients suffering from migraine and epilepsy but, probably, do not exclude a feedback in this event hierarchy and the deafferentation probability conditioned by a loss of hippocampal bonds. This state is confirmed by decreasing tracts in limbic zones that also plays an important role in the damage of brain extrahippocampal parts.
Thus, hippocampus is one of the main structures in disintegrating neuronal combinatorics and forms peculiarities of clinical manifestations of paroxysmal states, involving these or those brain areas at these two diseases.
Цель. Целью нашего исследования являлось сопоставление микроструктурных и нейрометаболических нарушений мозга, а также нейропатофизиологических особенностей у пациентов, страдающих от эпилепсии и мигрени. Методы. Нами обследовано 60 пациентов с мигренью в возрасте 16–42 лет (средний возраст составил 29,30 ± 0,52 года) и 60 — с эпилепсией в возрасте 18–51 года (средний возраст 28,20 ± 0,98 года). Шестидесяти пациентам с мигренью и 60 — с эпилепсией проведена диффузионная тензорная магнитно-резонансная томография. Протонная магнитно-резонансная спектроскопия выполнена 18 пациентам с мигренью и 28 — с эпилепсией. Все пациенты обследованы с использованием компьютерной электроэнцефалографии. Диагноз мигрени и эпилепсии был выставлен согласно международным рекомендациям. Результаты. Полученные данные не только указывают на повреждение нейрональной ткани у пациентов с мигренью и эпилепсией, но и, возможно, не исключают обратную связь в этой иерархии событий и вероятность деафферентации, обусловленной потерей гиппокампальных связей. Данное обстоятельство подтверждается уменьшением трактов в лимбических зонах, что играет важную роль также в повреждении экстрагиппокампальных отделов мозга.
Таким образом, гиппокамп является одной из главных структур в дезинтеграции нейрональной комбинаторики, а включение тех или иных областей мозга обусловливает особенности клинических проявлений пароксизмальных состояний при этих двух заболеваниях.
Мета. Метою нашого дослідження було порівняння мікроструктурних і нейрометаболічних порушень мозку, а також нейропатофізіологічних особливостей у пацієнтів, які страждають від епілепсії і мігрені. Методи. Нами обстежено 60 пацієнтів iз мігренню віком 16–42 роки (середній вік становив 29,30 ± 0,52 року) і 60 — з епілепсією віком 18–51 рік (середній вік 28,20 ± 0,98 року). Шістдесяти пацієнтам iз мігренню і 60 — з епілепсією проведена дифузійна тензорна магнітно-резонансна томографія. Протонна магнітно-резонансна спектроскопія виконана 18 пацієнтам iз мігренню і 28 — з епілепсією. Усі пацієнти обстежені з використанням комп’ютерної електроенцефалографії. Діагноз мігрені і епілепсії був виставлений згідно з міжнародними рекомендаціями. Результати. Отримані дані не тільки вказують на пошкодження нейрональної тканини у пацієнтів iз мігренню і епілепсією, а й, можливо, не виключають зворотний зв’язок у цій ієрархії подій і ймовірність деаферентації, обумовленої втратою гіпокампальних зв’язків. Ця обставина підтверджується зменшенням трактів у лімбічних зонах, що відіграє важливу роль також у пошкодженні екстрагіпокампальних відділів мозку.
Таким чином, гіпокамп є однією з головних структур у дезінтеграції нейрональної комбінаторики, а включення тих чи інших областей мозку обумовлює особливості клінічних проявів пароксизмальних станів при цих двох захворюваннях.
epilepsy, migraine, diffusion tensor magnetic resonance imaging with tractography, proton spectroscopy.
эпилепсия, мигрень, диффузионная тензорная магнитно-резонансная томография с трактографией, протонная спектроскопия.
епілепсія, мігрень, дифузійна тензорна магнітно-резонансна томографія iз трактографією, протонна спектроскопія.
Статья опубликована на с. 12-18
Migraine and epilepsy are comorbid neurological diseases possessing a number of common clinical and pathophysiological manifestations including a positive effect of taking anticonvulsants. The probability of migraine and epilepsy comorbidity is in developing the common pathophysiological changes leading to hypersynchronizing the neurons of central nervous system. Assessing and understanding the common mechanisms of these diseases allows justifying the possibility and prospects of therapeutic measures.
Epilepsy increases the risk of migraine in 2.4 times, and migraine increases the risk of developing epilepsy in 4.1 times [25]. The comorbidity of migraine and epilepsy significantly aggravates these diseases courses, including the resistance development. The existence and mutual effect of these pathological manifestations let indentify such a state as «migraine — epileptic seizure trigger» or migrilepsy in the International Classification of Diseases.
The main stage of diagnostics is a clinical study where the leading role is played by electroencephalography (EEG) that allows identifying abnormalities, including epileptiform activity which may be registered both in migraine and, to a greater extent, in epilepsy. A certain complexity of EEG–diagnosis is due to the fact that the epileptiform activity is observed in a number of neurological patients without epilepsy, as well as in 1–3 % of normal individuals. This fact is reflected in the International nomenclature recommended term «epileptiform activity» which has a probabilistic nature of its connection with epilepsy.
The common pathophysiological changes that lead to the hyperexcitability of neurons and to the development of such a pathological condition as hypersynchronization, under–lying a number of key states of migraine and epilepsy course, need to be clarified. These states should be differentiated, as a definite pathophysiological condition corresponds to each of them with the context of hypersynchronous activity reactions.
Despite the known causes of seizures and mechanisms of their implementation as well as the factors provoking paroxysmal states, neuroplasticity, being a consequence of brain response to the run pathological reactions, has a lea–ding role in this process [16]. In implementing seizures, one of intermediate links, and in a number of cases it is a domina–ting one, is a functional fitness of certain hippocampus parts and key neuroplasticity processes running in it, that is the «anticipatory» inhibition and «feedback» inhibition. The «anticipatory» inhibition provides an excitation spread on inhibitory interneurons by collaterals, blocking the development of hippocampus granular cell hypersynchronization [14]. The «feedback» inhibition arises at sending an impulse from a granular cell to an inhibitory interneuron that, in its turn, provides control over hypersynchronous excitation.
The presence of a low threshold of readiness for convulsions imparts an important role in developing paroxysmal conditions to hippocampus. Hippocampal commissure bombing is transmitted to thalamic and septal nuclei and thence back to hippocampus, with the limbic system of an opposite hemisphere and the brain as a whole involved [21]. Moreover, medial cortex, olfactory systems, thalamic intralaminar and rostal nuclei, lateral geniculate bodies, nuclei of pons cerebelli and mesencephalon raphe are projected in hippocampus. In its turn, hippocamp is projected into preoptical cortex, hypothalamic nonspecific nuclei, and thalamic median centre. In a similar functional organization, with hippocampus being a leading link, any afferent hypersynchronous stimulation will potentiate excitative processes and contribute to their further spreading [3]. This, it its turn, results in secondary temporalization of paroxysmal conditions which before had a nontemporal or generalized nature. Thus, hippocampus provides a harmonic correlation of inhibitory and excitative processes in brain.
The main mechanisms of forming hyperexcitable neural paths are determined to be an «excitable» axonal sprouting, decreasing the number of inhibitory neurons, reducing the «control» over neurons excited thereby and canalopathy which are due to the impairment of gene regulation of synaptic and neuronal plasticity resulting in cell loss, proliferation and inflammatory immune response [14].
The nervous system has an essential safety factor and quickly changes its structure and function due to the necessity to adapt to new conditions. Neuroplasticity is a phenomenon to optimize the performance of neural networks, the essence of which is to change the number of cells in brain and their functions (mainly, due to synaptic remodelling), depending on various factors of external and internal environment. Moreover, all these dynamic processes must be balanced for a normal function of a body as a whole, thus forming a homeostatic neuroplasticity [13, 17, 29]. The neuroplasticity appears to us as an own safety system of brain, and of course, of the whole body, which keeps it running [4].
The mechanisms and resources of neuroplasticity in depression and epilepsy are currently under study. The aspects of studying epilepsy are unique in this respect as all the levels of biological structure of brain are rearranged in this disease, describing the possibility of its adaptation to new conditions.
The existence of the concept of neuronal hyperexcitability in migraine is associated with discovering the substrate of inherited hemiplegic migraine type 1 which is characterized by a change of neuron calcium channels resulting in an excessive release of neurotransmitters, in particular, excitatory amino acids.
Thus, at functional magnetic resonance imaging (MRI) using visual stimulation of an occipital lobe, we identified depression foci that gave evidence of a slowly propagating wave of neurons and a glial cells depolarization replaced by a successively developing prolonged decrease in the neuronal activity and metabolism [18]. The phenomenon of spreading depression can occur not only in cortex, but also in other brain structures and, in particular, in hippocampus. At pre–sent, the role of cortical depression spread (CDS) as a trigger of migraine manifestations is decisive. In its turn, the CDS development activates a number of structures and provokes a dilatation of meningeal arteries which results in plasma extravasation and neurogenic inflammation, stimulates a production and a release of calcitonin gene–related peptide and nitric oxide [20]. The variability of clinical forms and first epilepsy episode time gives evidence of long–term changes progressing in the nervous system, known as epileptogenesis being an interesting natural model of neuroplasticity [26].
The basis of epileptogenesis is phenomena of apoptosis and synaptic reorganization lasting a certain period before the first clinical epileptic seizure. In particular, it should be noted that synaptic rearrangement leads to long–term and lasting changes in the efficiency of synapses (epileptic long–term potentiation). In a normal brain, girus dentatus cells provide a highly resistant filter for incoming signals from entorhinal cortex to hippocampus. In epilepsy, there is a structural remodelling of mossy epithelium synapses and forming aberrant synaptic contacts in girus dentatus followed by the excitation reverberation reducing a threshold of granule cell synchronization and by the loss of hilary interneurons. Such changes extend synaptic potentials and play an important role in epileptogenesis.
The neuropathophysiological views of the mechanisms of migraine and epileptic paroxysm naturally require studying microstructural brain lesions being a basis for developing a disease. Neuroimaging is an integral part of diagnostics letting localize a pathological process, determine etiological and syndromal diagnoses, assess the policy of medical measures. The use of routine computed tomography and MRI not always determines the morphological substrate underlying these diseases. Therefore neuroimaging functional techniques have become important, they contribute to new knowledge of migraine and epilepsy pathophysiology.
Our ideas of migraine have recently been transformed from vascular, neurovascular disorders into a nervous system damage. Functional neuroimaging has confirmed the fundamental nature of a spreading cortical depression as a pathophysiological mechanism of migraine’s aura. The studies of migraine let suggest that the pathophysiological generator was located in brain stem, besides, the involvement of cerebral peduncles, locus coeruleus, medial longitudinal fascicle and brainstem tegmentum in a pathological process was found.
Recent data on the migraine and epilepsy neuropathophysiological mechanisms require introducing new sensitive enough and specific methods of investigation. Diffusion tensor magnetic resonance imaging (DT MRI) is such a method, it is based on measuring the magnitude and direction of the diffusion of water molecules in the brain. This technique lets obtain a three–dimensional reconstruction of white matter fibers, assess pathway damage and build a virtual fiber path (tractography) [15, 16, 19]. The diffusion tensor values allow us to calculate some scalar values showing cell integrity: the mean diffusi–vity (MD) and fractional anisotropy (FA). Proton magnetic resonance spectroscopy (1H–MRS) belongs to the methods of functional neuroimaging. The basis of this method is the effect of «chemical shift» of resonance frequencies of hydrogen nuclei of a chemical compound in regard to a proton frequency in a water molecule. It permits to identify and to determine concentrations of various metabolites in any brain part intravitally [11, 27]. The change in these indicators generally reflects the mitochondria work and the level of neuron viability, the membrane integrity and the condition of cell energy system that is assessed as a marker of cell integrity [8, 11, 12, 22].
Material and Methods
We investigated 60 patients suffering from migraine, aged 16–42 years (mean age was 29.30 ± 0.52 years) and 60 — with epilepsy, aged 18–51 years (mean age was 28.20 ± ± 0.98 years). 60 patients with migraine and 60 with epilepsy underwent DT MRI.
The MRI studies were performed with a tomograph Philips with the magnetic field intensity of 1.5 T using a head coil consisting of 18 elements. The investigation protocol included standard programmes for brain MRI as well as target neuroimaging mediobasal parts of temporal lobes in fine scans with possible postprocessing and imaging in various areas. Among special programs, there were used fast spin–echo T2–weighted, perpendicular to the longitudinal axis of the hippocampus with a slice thickness of 2 mm, and 3D T1 SP6R pulse sequences with reforming and obtaining images of temporal lobe mediobasal areas, perpendicular and parallel to the longitudinal axis of hippocampus. The diffusion tensor was calculated for each voxel resulting in getting images (map) of FA and MD. The tractography was performed for all the examined, assessing the number of path lines for front and back quadrants in axial scans. Visual identifying the obtained tracts (tractography results) was performed on the basis of the MRI–map for brain white substance [23]. The indices of FA and MD were calculated at similar sections for all the images obtained. The MRI results were grouped according to a side of lateralization of an epiactivity focus.
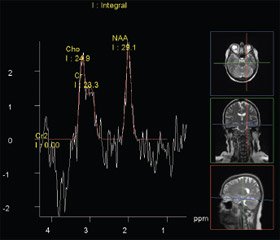
18 migraine and 28 epilepsy patients underwent 1H–MRS. The proton MRS is a unique method of obtaining information about neurochemical processes in the brain of patients with epileptic seizures. The basis of this method is the effect of «chemical shift» — a change of the resonance frequency of protons belonging to various chemical compounds. The differences among frequencies of individual spectral peaks are presented by a millionth part regarding to a control signal with a known resonance frequency [23] that corresponds to the indices of a metabolite chemical shift, the peaks of which are determined in vivo in a proton spectrum. In normal people, each anatomical structure is characterized by a stable concentration of major metabolites. In brain, the peaks of N–acetylaspartate (NAA — 2.00 ppt), choline (Cho — 3.22 ppt) and сreatine (Cr — 30.03 and 3.94 ppt) are the most evident ones. N–acetylaspartate is a marker of an active neuron, and its signal is the most intense in the spectrum. The role of NAA in the metabolism of nerve tissue is not still defined but its level decreases in many diseases occurring in a neuronal integrity disorder. The signal from choline is a total quantity of the choline stock in brain and is a structural element of complex lipids. Choline is a marker of increasing the cell membrane metabolism, being a characteristic of its damage.
Proton MRS provides a unique opportunity to determine the anatomical localization of metabolic processes defining qualitative and quantitative indicators as well to objectify treatment outcome control. The clinical investigations suggest that 1H–MRS is maximum sensitive for detecting metabolic changes in studying paroxysmal states.
All patients were examined using the computer EEG. Migraine and epilepsy were diagnosed according to International Recommendations. 18 normal volunteers were a control for conducting diffusion tensor MRI.
The statistical analysis was performed using the program Statistica 6.0. We used the Mann–Whitney test to assess the significance of differences of intergroup indicators; the correlation analysis was performed using the Spearman’s coefficient. Sample differences were considered significant at a significance level p < 0.05.
Results
The results of MRI investigations in patients with epilepsy revealed a dilatation of subarachnoid spaces practically in all of them (97.8 %), in 63.0 % — above temporal lobes of brain, with pathological change prevailing on the side of a focus recorded by EEG in 96.4 %. Significant enlargements of third and forth ventricles were detected in 83.3 and 90.5 % of cases, respectively. Small gliosis foci and cysts in pale globe and thalamus were revealed in 13 % of patients.
Conducting diffusion tensor MRI with tractography made possible to determine microstructural changes in all patients examined [9]. FA index was the most significant one. This index was significantly changed in the temporal lobe structures (p < 0.05), mainly in epifocus hemisphere. The MD values appeared to be fine enough in detecting microstructural changes that were more evident in the group of pharmacoresistant epilepsy patients. The epilepsy compensated and with a remission of more than 1 year was characterized by a slight decrease in this indicator compared with the norm. This index may be a predictor of prognosing a disease clinical course, whereas the FA values in patients, having PRE and a remission, did not significantly differ (p > 0.05). One should note a certain connection of MD indices of the front and back parts of hemispheres with the sizes of lateral and third ventricles on an epileptic focus side. In the presence of an epileptic focus, there is a correlation of a FA decrease and a MD increase with a seizure severity factor. The obtained results can characterize the efficacy of a therapy; however, this requires further investigation. Studying the tractography pattern showed that a pathway depletion corresponded to a decrease in FA and an increase in MD, mainly on a paroxysmal activity side. Besides, we detected the areas of altered white matter not only in an epileptogenic hemisphere but in an opposite one [2, 5, 10, 24, 28].
The results of proton spectroscopy revealed a decrease in the most important correlation of NAA/(Cho + Cr) less 0.71 in hippocampus on the side of epileptic focus in the half of patients and in the other half — on both sides. The concentration of neurometabolites in the external parts of temporal lobes altered mainly on the side of an epileptogenic focus determined by EEG–mapping. Comparing 1H–MRS results with DT MRI data showed a correlation of low NAA/(Cho + + Cr) indices in the external parts of temporal lobes with the nonvisualization of brain anterior and posterior commissures (r = 0.8; p = 0.009), indicating the significance of the normal state of brain white substance passers providing a cortical parts optimal functioning [6, 10] (Fig. 1).
The analysis of diffusion tensor magnetic resonance ima–ging data revealed a number of specific changes in migraine patients depending on a clinical manifestation — the presence or the lack of aura.
According to the diffusion tensor magnetic resonance ima–ging, the patients without aura were characterized by structural abnormalities with a decrease in FA index in the anterior frontal and temporal lobes of brain and by significant MD changes in the posterior parts of cerebral hemispheres (p > 0.05).
Besides, the tractographic pattern was depleted in brain occipital lobes, and the posterior commissure was not visualized. The EEG picture was characterized by slow wave and photoparoxysmal activity prevailing [7]. According to the EEG–mapping, significant changes in the bioelectric activity were revealed in these patients. That was manifested in decreasing the alpha rhythm power and in inversing its frequency–spatial structure, in increasing the power of slow range waves, and in photoparoxysmal activity (Fig. 2).
Significant fractional anisotropy decreasing and mean diffusivity increasing in patients with aura was noted in the area of optic radiation and hippocampal connections. These decrease and increase limit the deafferentation of brainstem structures. These changes got combined with the hyperexcitability or hypersynchronous blink reflex recorded on the side of hemicranias (p < 0.05). As well, we should mention a significant rate of paroxysmal activity recording, mainly in temporooccipital leads. It was accompanied by a decrease in the alpha rhythm power and an increased index of slow–wave activity [7]. The EEG spectral analysis indicates that the phenomenon of spreading depression can occur not only in the cortical areas, but also in other structures, particularly in hippocampus and cerebellum. This phenomenon is accompanied by edema developing, various neurotransmitters and neuromodulators releasing, and a massive flow of potassium ions from cells [1].
Taking into account the available pathophysiological data, the study of neurochemical processes giving an opportunity to determine the anatomical localization of metabolic changes in the brain structures of hippocampus, thalamus, cingulate gyrus, frontal and occipital regions was carried out. Significant changes in the NAA/(Cho + Cr) correlation in hippocampus were recorded in all patients, mainly on the side of a hemicranias localization, with being more marked in a comorbid aura seizure that ranged from 0.29 to 0.64 (normal — more 0.71). Similar changes were recorded for NAA/Cr и Cho/Cr correlations (Fig. 3А).
As for changes in the correlation of neurometabolites in thalamus, there was a downward tendency for all indices only in migraine with aura (Fig. 3B). Migraine without aura was characterized by Cho/Cr decreasing without changing other metabolite concentration.
The analysis of cingulate gyrus neurometabolite concentrations revealed changes of another trend which was in all indices decreasing in migraine without aura.
The change in the correlations of neurometabolites in a frontal lobe in migraine without aura and significant Cho/Cr changes in migraine with aura attract our attention.
Studying the investigated occipital lobe indices revealed their decrease in patients with aura on a hemicranias side which was similar to changes in the hippocampal complex (p < 0.05).
Discussion
The obtained data let confirm a common pathophysiolo–gical mechanism of developing paroxysmal states manifested in a pathological hypersynchronous activity. The common functional changes resulting in a neuronal hyperexcitability are a probable cause of migraine and epilepsy comorbidity. The phenomenon of spreading depression appearing not only in cortex but in other brain structures, first of all in hippocampus, is an important element in this process. In this structure, we detected significant changes of metabolites, in the chain of their spreading and influencing other brain structures, and first of all, thalamic structures that limits the manifestation of developing thalamocortical dysfunction. Certain changes in a tract configuration, their integrity and genetic adequacy are a confirmation there of. The hippocampus having initially a low threshold of convulsive readiness potentiates excitative processes and contributes to their further spreading.
Conclusions
The obtained data indicate not only a neuron tissue lesion in patients suffering from migraine and epilepsy but, probably, do not exclude a feedback in this event hierarchy and the deafferentation probability conditioned by a loss of hippocampal bonds. This state is confirmed by decreasing tracts in limbic zones that also plays an important role in the damage of brain extrahippocampal parts.
Thus, hippocampus is one of the main structures in disintegrating neuronal combinatorics and forms peculiarities of clinical manifestations of paroxysmal states, involving these or those brain areas.
Modern methods of functional neuroimaging provide data underlying the disease, let retrace clinical courses of paroxysmal conditions and determine the correlation of a clinical course, neurophysiological outcomes and structural–metabolic disorders.
All this demands to continue studies and to identify diagnostic criteria and the efficacy of a performed therapy.
1. Азимова Ю.Э. Мигрень и эпилепсия / Ю.Э. Азимова, Г.Р. Табеева // Эпилепсия и пароксизмальные состояния. — 2009. — № 1. — С. 21–25.
2. Диффузионная тензорная магнитно–резонансная томография и трактография в оценке проводящих путей у пациентов с эпилепсией / В.В. Евстигнеев, О.В. Кистень, И.В. Булаев, Р.А. Сакович // Вестник Казахского национального медицинского университета. — 2012. — С. 19–21.
3. Зенков Л.Р. Хипокампальная формация / Л.Р. Зенков // Нейрофизиология / Под ред. Ив.К. Георгиева, А.М. Вейна. — София: Медицина и физкультура, 1987. — С. 109–117.
4. Кистень О.В. Нейропластичность — парадигма эпилептологии / О.В. Кистень // Мед. панорама. — 2010. — Т. 5, № 113. — С. 3–8.
5. Кистень О.В. Транскраниальная магнитная стимуляция в эпилептологии / О.В. Кистень, В.В. Евстигнеев. — Вильнюс: Grafija, 2013. — 368 с.
6. Магнитно–резонансная трактография и спектроскопия в оценке микроструктурных изменений головного мозга при эпилепсии / И.В. Булаев, О.В. Кистень, Р.А. Сакович, В.В. Евстигнеев // Материалы научно–практической конференции «Актуальные вопросы лучевой диагностики», г. Минск, 8–9 ноября 2012 г. / БелМАПО; под ред. Ю.Ф. Полойко. — Минск, 2012. — С. 57–60.
7. Морфо–функциональные особенности клинического проявления мигрени / В.В. Евстигнеев, К.А. Садоха, О.В. Кистень, Р.А. Сакович // Материалы XV Международной конференции «Основные направления фармакотерапии в неврологии», г. Судак, 24–26 апреля 2013 г. / Под. ред. С.М. Кузнецовой. — К., 2013. — С. 264–265.
8. Одинак М.М., Базилевич С.Н. Возможности и опыт применения методов нейровизуализации при эпилепсии // Эпилептология в медицине XXI века / Под. ред. Е.И. Гусева, А.Б. Гехт. — М.: ЗАО «Светлица», 2009. — 572 с.: ил. — С. 287–297.
9. Опыт применения диффузионной тензорной магнитно–резонансной томографии в морфологической диагностике эпилепсии / В.В. Евстигнеев, О.В. Кистень, И.В. Булаев, Р.А. Сакович // Материалы Республиканской научно–практической конференции с международным участием «Пароксизмальные состояния у детей и взрослых», г. Витебск, 22–23 ноября 2012 г. // Неврология и нейрохирургия. Восточная Европа; гл. ред. С.А. Лихачев. — № 4 (16). — С. 97.
10. Особенности структурных изменений белого вещества мозга в клинической реализации эпилепсии / О.В. Кистень, В.В. Евстигнеев, Р.А. Сакович, И.В. Булаев // Эпилепсия и пароксизмальные состояния. — 2013. — Т. 5, № 1. — С. 15–22.
11. Труфанов Г.Е. Магнитно–резонансная спектроскопия: Руководство для врачей / Под ред. Труфанова Г.Е., Тютина Л.А. — СПб.: ЭЛБИ–СПб, 2008. — 239 с.
12. Тютин Л.А. Протонная магнитно–резонансная спектроскопия в диагностике заболеваний головного мозга / Л.А. Тютин, А.В. Поздняков, Л.А. Стуков // Вестник рентгенологии и радиологии. — 1999. — № 5. — С. 4–7.
13. Харченко Е.П. Пластичность мозга / Е.П. Харченко, М.Н. Клименко // Химия и жизнь. — 2004. — № 6. — С. 26–31.
14. Babb T.L. Pathological findings in epilepsy / T.L. Babb, W.J. Brown // Surgical treatment of the epilepsies / Ed. by J. Engel Jr. — New York, 1987. — Р. 511–540.
15. Basser P.J., Pajevic S., Pierpaoli C. et al. In vivo fiber tractography using DT–MRI data // Magn. Reson. Med. — 2000. — 44. — 625–632.
16. Catani M., Howard R.J., Pajevic S., Jones D.K. Virtual in vivo interactive dissection of white matter fascicule in the human brain // NeuroImage. — 2002. — Vol. 17. — P. 77–94.
17. Chakraborty R. Neuroplasticity — a paradigm shift in neurosciences / R. Chakraborty [et al.] // J. Indian Med. Assoc. — 2007. — Vol. 105, № 9. — P. 513–521.
18. Charles A., Brennan K.C. Cortical spreading depression — new insights and persistent questions // Cephalalgia. — 2009. — Vol. 29. — P. 1115–1124.
19. Ciccarelli O., Catani M., Johansen–Berg H., Clark C., Thompson A. Diffusion–based tractography in neurological disorders: concepts, applications, and future developments // Lancet Neurol. — 2008. — Vol. 7. — P. 715–727.
20. Hadjikhani N., Sanchez Del Rio M., Wu O. et al. Mechanisms of migraine aura revealed by functional MRI in human visual cortex // Proc. Natl Acad. Sci. USA. — 2001. — Vol. 98. — P.4687–4692.
21. Izquierdo I. Hippocampal physiology: experiments of regulation of its electrical activity, on the mechanisms of seizures, and on a hypothesis of learning // Behav. Biol. — 1972. — Vol. 5. — P. 669–698.
22. Maton B.M. Proton MRS: N–acetylaspartate, creatine, choline / B.M. Maton, R.I. Kuzniecky // Adv. Neurol. — 2000. — Vol. 83, № 2. — P. 253 –259.
23. Moris W.S. MRI atlas of human white matter / W.S. Moris, L.M. Nagae–Poetscher, P.C.M. Van Zijl. — 2nd Ed. — Amsterdam, Netherlands: Elsevier, 2011. — 255 p.
24. Peculiarities of structural white matter abnormalities on clinical realization of epilepsy / V. Kistsen, V. Evstigneev, I. Bulaev, R. Sakovich // Journal of the Neurological Sciences. — 2013. — Vol. 333, Suppl. 1. — P. e23.
25. Ramsey N.F. Proton–Proton Scattering at 105 Mev and 75 Mev / N.F. Ramsey, R.W. Birge, U.E. Kruse // Phys. Rev. — 1951. — Vol. 83. — P. 274.
26. Scharfman H.E. Epilepsy as an example of neural plasti–city / H.E. Scharfman // Neuroscientist. — 2002. — Vol. 8, № 2. — P. 154–173.
27. Silberstein S.D., Lipton R.B., Goatsby P.J. Headache in clinical practice. — London: Martin Dunitz, 2002. — 211 p.
28. The effect of structural white matter abnormalities on the clinical course of epilepsy / V.V. Evstigneev, V.V. Kistsen, I.V. Bulaev, R.A. Sakovich // Adv. Clin. Exp. Med. — 2013. — Vol. 22, № 4. — P. 529–537.
29. Turrigano G. Homeostatic plasticity in the developing nervous system / G. Turrigano, S. Nelson // Nat. Neurosci. Rev. — 2004. — Vol. 5. — P. 97–107.
30. Vion–Dury J., Salvan A.M., Cozzone P.J. Proton magnetic resonance spectrometry for the non–invasive exploration of human brain metabolism: current and future clinical applications // Rev. Neurol. — 1999. — Vol. 155, № 11. — P. 903–926.

/15/15.jpg)
/16/16.jpg)