Международный неврологический журнал №5 (107), 2019
Вернуться к номеру
Опитувальник щодо інтимного життя та сексуальності у хворих на розсіяний склероз: валідизація та адаптація для україномовного населення
Авторы: O.I. Nehrych (1), V.I. Pyrohova (1), J.G. Portnoy (2), M. Stimmel (2), F.W. Foley (2), T.I. Nehrych (1)
1 - Danylo Halytsky Lviv National Medical University, Lviv, Ukraine
2 - Ferkauf Graduate School of Psychology, Yeshiva University, New York, USA
Рубрики: Неврология
Разделы: Клинические исследования
Версия для печати
Актуальність. Сексуальна дисфункція є поширеною проблемою в жінок із розсіяним склерозом (РС). Опитувальник щодо інтимного життя та сексуальності у хворих на розсіяний склероз (MSISQ-19) — це специфічний інструмент оцінки статевої дисфункції у хворих на РС. Мета дослідження — перекласти та перевірити валідність української версії опитувальника MSISQ-19. Матеріали та методи. Переклад оригінальної версії MSISQ-19 проводився відповідно до стандартної процедури. Опитувальник був заповнений 126 жінками зі встановленим діагнозом РС. Внутрішню узгодженість оцінювали за допомогою показника альфа Кронбаха. Конвергентну валідність встановлювали за допомогою кореляції з опитувальником якості життя у хворих на РС (MSQOL-54) та розширеною шкалою оцінки інвалідизації (EDSS). Результати. Загальна надійність шкали (α Кронбаха = 0,943) та надійність для первинного (α Кронбаха = 0,901), вторинного (α Кронбаха = 0,875) та третинного (α Кронбаха = 0,918) субтестів були високими. Рівень інвалідності, який вимірювали за допомогою EDSS, та якість життя, оцінена за допомогою MSQOL-54, вірогідно корелювали із загальним балом MSISQ-19. Субтести оцінки якості життя, пов’язаної з фізичним та психічним здоров’ям, суттєво корелювали із загальним балом MSISQ-19, а також з первинною, вторинною та третинною сексуальною дисфункцією. Задоволення сексуальною функцією суттєво корелювало з загальним балом MSISQ-19, а також із первинною, вторинною та третинною сексуальною дисфункцією. Висновки. Результати дослідження показують, що українська версія MSISQ-19 є надійним та дієвим інструментом оцінки статевої функції у жінок із РС. Опитувальник може використовуватися під час планового консультування, щоб виявити сексуальну дисфункцію, забезпечити лікування, профілактику погіршення статевої функції і, таким чином, зберегти належну якість життя хворих із РС.
Актуальность. Сексуальная дисфункция является распространенной проблемой у женщин с рассеянным склерозом (РС). Опросник интимной жизни и сексуальности у больных рассеянным склерозом (MSISQ-19) — это специальный инструмент для оценки сексуальной дисфункции у пациентов с РС. Цель исследования — перевести и проверить валидность украинской версии MSISQ-19. Материалы и методы. Перевод оригинальной версии MSISQ-19 проводился в соответствии со стандартной процедурой. Опросник был заполнен 126 женщинами с подтвержденным диагнозом РС. Внутренняя согласованность оценивалась с использованием показателя альфа Кронбаха. Конвергентная валидность была установлена с использованием корреляции с опросником качества жизни у больных с РС (MSQOL-54) и расширенной шкалой оценки инвалидизации (EDSS). Результаты. Общая надежность шкалы (α Кронбаха = 0,943) и надежность для первичного (α Кронбаха = 0,901), вторичного (α Кронбаха = 0,875) и третичного (α Кронбаха = 0,918) субтестов были высокими. Уровень инвалидности, измеренный с помощью EDSS, и качество жизни, определенное по MSQOL-54, достоверно коррелировали с общим показателем MSISQ-19. Субтесты оценки качества жизни, связанного с физическим и психическим здоровьем, достоверно коррелировали с общим баллом MSISQ-19, а также с первичной, вторичной и третичной сексуальной дисфункцией. Удовлетворение сексуальной функцией в значительной степени коррелировало с общим показателем MSISQ-19 и с первичной, вторичной и третичной сексуальной дисфункцией. Выводы. Результаты исследования показывают, что украинская версия MSISQ-19 является надежным и действенным инструментом для оценки сексуальной функции у женщин с РС. Его можно использовать во время повседневного консультирования, чтобы выявить сексуальную дисфункцию, обеспечить лечение, профилактику ухудшения сексуальной функции и, следовательно, сохранить надлежащее качество жизни пациентов с РС.
Background. Sexual dysfunction is a common problem in women with multiple sclerosis (MS). The Multiple Sclerosis Intimacy and Sexuality Questionnaire-19 (MSISQ-19) is a specific instrument to evaluate sexual dysfunction in MS patients. The purpose of the study was to translate and validate the Ukrainian version of the MSISQ-19. Materials and methods. The original version of MSISQ-19 was translated under a standard procedure. A sample of 126 females with MS completed the questionnaire. Internal consistency was evaluated using Cronbach’s alpha. Convergent validity was established using correlation with the Multiple Sclerosis Quality of Life-54 (MSQOL-54) and the Expanded Disability Status Scale (EDSS). Results. Total scale reliability (Cronbach’s α = 0.943) and reliability for the primary (Cronbach’s α = 0.901), secondary (Cronbach’s α = 0.875), and tertiary subscales (Cronbach’s α = 0.918) were high. Disability level, measured with the EDSS, and quality of life, measured with the MSQOL-54, significantly correlated with MSISQ-19 total score. Both physical and mental health-related quality of life on the MSQOL-54 significantly correlated with MSISQ-19 total score, and with primary, secondary, and tertiary sexual dysfunction. Satisfaction with sexual function significantly correlated with MSISQ-19 total score, and with primary, secondary, and tertiary sexual dysfunction. Conclusions. The study findings suggest that the Ukrainian version of the MSISQ-19 is a reliable and valid instrument for sexual function assessment in Ukrainian women with MS. It can be used during routine counseling to introduce the theme of sexuality, to detect sexual dysfunction, provide treatment, and prevent the development of more severe problems and therefore preserve the proper quality of life for MS patients.
розсіяний склероз; опитувальник щодо інтимного життя та сексуальності у хворих на розсіяний склероз; сексуальна функція
рассеянный склероз; опросник интимной жизни и сексуальности у больных рассеянным склерозом; сексуальная функция
multiple sclerosis; Multiple Sclerosis Intimacy and Sexuality Questionnaire-19; sexual function
Introduction
Multiple sclerosis (MS) is a chronic immune–mediated neurodegenerative disease of the central nervous system [1]. The first symptoms of MS typically occur between 20 and 40 years with a distinct female preponderance (3 : 1 female to male ratio during reproductive ages) [2]. Most researchers suggest that MS has a negative impact on patients’ sexual life, and their satisfaction with the relationship is lower than in the general population [3]. Young women affected by MS are challenged with finding a partner, building relationships, creating families and having sex. Moreover, sexual dysfunction may interfere with family planning and fertility.
According to different studies, the rate of sexual problems among female MS patients varies between 40 and 80 %, depending on the severity of disability of the group studied and duration of their illness [4]. Sexual dysfunction can occur in patients with early disease and has even been reported in newly diagnosed women [5].
A conceptual model for sexual problems in MS in terms of primary, secondary or tertiary sexual dysfunction was proposed to characterize three levels of influence. Primary sexual dysfunction results from MS–related demyelinating lesions in the spinal cord and/or brain that directly impair sexual fee–lings or responses. It includes a decrease in libido, reduced genital sensation, a lack of lubrication, and a decrease in intensity and frequency of orgasms depending on genital nerve damage caused by MS. Secondary sexual dysfunction is related to physical disorders concerning MS and side effects of drugs. They indirectly influence the sexual response. It is caused by fatigue, attention and concentration disorders, difficulties with mobility, impairment of coordination, muscle stiffness, bladder and bowel dysfunctions, muscle weakness, lower extremity spasms, tremor, pain, and adverse effects of the MS drugs. Tertiary sexual dysfunction is associated with psychological, emotional, social, and cultural aspects of MS that can interfere with sexual feelings and sexual response. Sexual dysfunction in this group arise from depression, anxiety, irritability, decreased self–respect, impaired body image perception, decreased feeling of sexual attractiveness, fear of being sexually rejected, difficulty in the relationship with partner, fear of dependency, and anxiety [6].
Despite the fact of the emerging awareness of the predominance of sexual dysfunction in MS patients, the majority of them are never questioned about sexual issues [7]. Patients often feel uncomfortable to raise their sexuality problems in the physician’s office, but most of them consider it to be appropriate for healthcare providers to address sexual functioning during routine visits [8]. Previous studies have demonstrated that sexual counseling and educational materials about sexual issues in MS are beneficial for the patients [9].
In order to provide effective counseling and management, it is necessary to use reliable, sensitive and valid screening tools for the assessing of sexual function at first. One of the most specific instruments to evaluate sexual dysfunction in MS patients is the Multiple Sclerosis Intimacy and Sexuality Questionnaire–19 (MSISQ–19) [10]. The questionnaire is self–completed and there is a risk that it might be misinterpreted across cultures and populations.
The purpose of the study. The present study purposed to translate the English version of the MSISQ–19 into Ukrainian using standard back–translation techniques and evaluate the reliability, construct validity, and concurrent validity of the Ukrainian version of the MSISQ–19 in a sample of women diagnosed with MS in Ukraine. There has previously been no instrument for assessing sexual function in MS patients who speak Ukrainian. It was expected that Ukrainian healthcare practitioners would receive an effective method for sexual function evaluation in MS and improve MS patients’ management.
Materials and methods
This work was approved by the regional Ethics Committee of Danylo Halytsky Lviv National Medical University (Protocol no. 5 from 23.02.2017) in accordance with the regulations of the Ministry of Health of Ukraine.
Two independent translators translated the original English version of MSISQ–19 scale into Ukrainian. The translators and one of the authors compared the two translations and established a single interim version. After that, two other professional translators translated the interim version into English [11]. Finally, an expert committee consisting of the researchers, translator, sexologist and neurologist reviewed all the translations. The final back–translated English version was evaluated based on semantic, idiomatic, experiential, and conceptual equivalence, and a final Ukrainian version of the MSISQ was produced. To ensure face validity, the final Ukrainian version was given to 28 patients who completed it and confirmed their understanding of the items.
Patients for the study were recruited from Lviv Regional Centre of Multiple Sclerosis, Lviv, Ukraine. Totally, 126 females aged from 18 to 60 years were included after the researchers explained the aim of the study and received a signed informed consent. The inclusion criteria were diagnosis of MS according to the McDonald diagnostic criteria, sexual activity, and a score of the Expanded Disability Status Scale (EDSS) less than 8 [12]. Exclusion criteria were history of major chronic disease, chronic alcohol consumption, and cognitive impairment. History of the participants was obtained, and their physical examination was performed.
This study used several measures: a study–specific questionnaire on demographic, clinical, and medical characteristics; the Multiple Sclerosis Quality of Life–54 (MSQOL–54); the EDSS; and the MSISQ–19. The MSISQ–19 and MSQOL–54 questionnaires were administered in a quiet and private space to minimize possible restraints. Data regar–ding age, level of education, disease duration, neurological examination, and EDSS score were collected. In order to analyze the stability and reliability of the scale, 33 randomly selected patients were asked to undergo retest in the period of 18–22 days after the first test.
EDSS is used to determine the neurological disabi–lity and functional capacity of MS patients. It evaluates the functions of eight systems (pyramidal, cerebellar, brainstem, vision, bladder and bowel, sensorial, and cerebral). The systems are scored according to the degree of its functional impairment. The EDSS score changing between 0 and 10 is obtained after the addition of restrictions in daily life to the scores of these functional systems [13].
Multiple Sclerosis Quality of Life–54 is the most–known disease–specific tool for assessing the quality of life in patients with MS. It was developed to combine the generic quality of life aspects of the Short Form Health Survey (SF–36) with MS–targeted dimensions and ratings for the overall quality of life. Therefore, 18 disease–specific items were added to the original 36 items of the SF–36. The 54 items are divided into 12 multi–item and 2 single–item scales. The MSQOL–54 items are linearly transformed to 0–100 scores and final scores are obtained by averaging items within the scales. Higher values indicate better functioning and well–being [14].
The MSISQ self–report questionnaire contains 19 items. This scale evaluates the impact of various MS symptoms on the patient’s sexual activity during the previous 6 months. It assesses three dimensions of sexual dysfunction: primary (five items), secondary (nine items), and tertiary (five items). Each item is rated on a five–point scale (1, never; 2, almost never; 3, occasionally; 4, almost always; 5, always). The original validation study of the MSISQ–19 demonstrated its reliability, construct, criterion, and concurrent validity on measures of marital satisfaction; MS–related neurological impairment and level of disability; psychological distress and well–being; and global sexual dysfunction [10].
Descriptive analyses were performed. Principal component analysis (PCA) was conducted after Kaiser–Meyer–Olkin statistic (KMO) was calculated and Bartlett’s test of sphericity was performed. Internal consistency was evaluated using Cronbach’s alpha and test–retest reliability was evaluated using intraclass correlation coefficient (ICC). Convergent validity was established using correlation with the MSQOL–54 and EDSS. Missing data were excluded from analyses on a pairwise basis. Data were statistically processed using the StatsDirect 3, Ver. 3.1.7 software package (StatsDirect Ltd, UK, no. 04399867).
Results and discussion
The sample included 126 women, all of whom were Caucasian and involved in a heterosexual relationship. Additio–nal characteristics of the sample are described in Table 1.
PCA was used to determine the component structure underlying the 19 items. Sampling adequacy was supported (KMO = 0.928) and Bartlett’s test verified assumption of homoscedasticity (p < 0.001). Due to intercorrelation of extracted components, an oblique promax rotation was applied to the component solution. Based on a criterion of eigenvalue greater than 1, a three–component solution emerged, corresponding to primary, secondary, and tertiary sexual dysfunction. Items were assigned to corresponding subscales based on the highest component loading. All items loaded onto the same subscales as in the English validation with the exception of item 8, “Problems moving my body the way I want during sexual activity,” which was re–assigned from the secondary dysfunction to the tertiary dysfunction subscale. The three–component solution explained 66.18 % of the total variance. Table 2 shows the results of the PCA, including component loadings ordered by theoretical construct.
Total scale reliability was high (Cronbach’s α = 0.943). Reliability was high for the primary (Cronbach’s α = 0.901), secondary (Cronbach’s α = 0.875), and tertiary subscales (Cronbach’s α = 0.918). For the 33 participants who completed a second MSISQ–19, test–retest reliability was strong (ICC = 0.991).
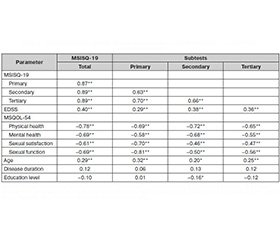
Pearson product–moment correlations were calculated to measure intercorrelation among the primary, secondary, and tertiary MSISQ–19 subscales and the total scale score. All subscales significantly intercorrelated, and each correlated significantly with the total scale score (p < 0.001 in all cases). Pearson correlations were also used to measure the strength of association between the MSISQ–19 and EDSS and MSQOL–54 scores to demonstrate convergent validity, as well as between the MSISQ–19 and age and disease duration. Kendall’s τb correlations were calculated to examine the strength of association between the MSISQ–19 and education level.
Disability level, measured by the EDSS, significantly correlated with MSISQ–19 total score (r = 0.40, p < 0.001), and with primary (r = 0.29, p = 0.002), se–condary (r = 0.38, p < 0.001), and tertiary sexual dysfunction (r = 0.36, p < 0.001). Physical health–related quality of life on the MSQOL–54 significantly correlated with MSISQ–19 total score (r = −0.78, p < 0.001), and with primary (r = −0.69, p < 0.001), secondary (r = −0.72, p < 0.001), and tertiary sexual dysfunction (r = −0.65; p <0.001). Mental health–related quality of life on the MSQOL–54 significantly correlated with the MSISQ–19 total score (r = −0.69, p < 0.001), and with primary (r = −0.58, p < 0.001), secon–dary (r = −0.68, p < 0.001), and tertiary sexual dysfunction (r = −0.55, p < 0.001). Satisfaction with sexual function significantly correlated with MSISQ–19 total score (r = −0.61, p < 0.001), and with primary (r = −0.70, p < 0.001), secon–dary (r = −0.46, p < 0.001), and tertiary sexual dysfunction (r = −0.47, p < 0.001). Sexual function significantly correla–ted with the MSISQ–19 total score (r = −0.69, p < 0.001), and with primary (r = −0.81, p < 0.001), secondary (r = −0.50, p < 0.001), and tertiary sexual dysfunction (r = −0.56, p < 0.001).
Age significantly correlated with the MSISQ–19 total score (r = 0.29, p = 0.001), and with primary (r = 0.32, p < 0.001), secondary (r = 0.20, p = 0.023), and tertiary sexual dysfunction (r = 0.25, p = 0.005). Disease duration did not significantly correlate with the MSISQ–19 total scale or subscales, and education level did not significantly correlate with the MSISQ–19 total score, primary subscale score, or tertiary subscale score, though it did show a modest but significant correlation with the secondary subscale score (τb = −0.16, p = 0.030). These results are summarized in Table 3.
Multiple sclerosis develops symptoms that can impact sexual function and satisfaction. The MSISQ–19 was made to evaluate the influence of MS symptoms on sexual activity and satisfaction and on the overall quality of intimate relationships [10].
Although patients with MS in the Ukrainian population have a high rate of sexual dysfunction, there are no measures validated in this group. It is reasonable to culturally adapt and validate MS–specific sexuality assessments, such as the MSISQ–19, in Ukraine. The results of this study confirmed construct validity of the Ukrainian version of the MSISQ–19 for primary, secondary, and tertiary sexual dysfunction, consistent with the original validation study [10].
Considering sexual dysfunction may result from MS–related symptoms that interfere with the sexual response, such as loss of coordination or tremor, bowel/bladder dysfunction, weakness or difficulty moving limbs, problems with speech, and numbness or loss of sensation, all of which are assessed by the EDSS, it was hypothesized that EDSS scores would correlate with the MSISQ–19 total scores and its subscales score. Our study showed that disability level, measured by the EDSS, significantly correlated with the MSISQ–19 total score, and with primary, secondary, and tertiary sexual dysfunction. Consequently, the MSISQ–19 can be used to assess MS symptoms and to verify the influence of MS symptoms on sexual functio–ning [10].
It was expected that the MSQOL–54 score would correlate with scores on the MSISQ–19 and its subscales, because sexual dysfunction is known to impact quality of life [15]. Both physical and mental health–related qua–lity of life on the MSQOL–54 significantly correlated with the MSISQ–19 total score, and with primary, secondary, and tertiary sexual dysfunction. Satisfaction with sexual function significantly correlated with the MSISQ–19 total score, and with primary, secondary, and tertiary sexual dysfunction. The EDSS and the MSQOL–54 confirmed the convergent validity of the MSISQ–19. Age significantly correlated with the MSISQ–19 total score and its subscales. Such an outcome was expected, because there is strong evidence that sexual activities and sexual function decline with age even in healthy population [16]. Therefore, age should be assessed as an independent factor in the development of sexual dysfunction.
Conclusions
1. The study findings suggest that the Ukrainian version of the MSISQ is a reliable and valid instrument for sexual function assessment in Ukrainian women with MS.
2. The Ukrainian version of the MSISQ–19 can be used in the daily care of MS patients.
3. The administration of a standardized instrument can facilitate the initial approach and encourage healthcare professionals to introduce the theme of sexuality during routine counseling.
4. The Ukrainian version of the MSISQ can be a valuable tool to detect sexual dysfunction, provide treatment, and prevent the development of more severe problems and therefore preserve the proper quality of life for MS patients.
Limitations of the study. Our sample consisted only of female patients with MS. Although the component structure and correlations with other measures found in this study corresponded to the English version, it remains uncertain whether the Ukrainian version is sensitive to interventions for sexual function improvement and to changes in disease status over time. Further studies are needed to establish psychometric properties for male MS patients in Ukraine.
Conflicts of interests. Authors declare the absence of any conflicts of interests that might be construed to influence the results or interpretation of their manuscript.
Funding. This work was supported by Danylo Halytsky Lviv National Medical University.
Ethical Approval. This work was approved by the regional Ethics Committee of Danylo Halytsky Lviv National Medical University (Protocol no. 5 from 23.02.2017).
Information about the authors’ contribution: Nehrych O.I. — collection and processing of the materials; analysis of data obtained, text writing; Pyrohova V.I. — conception and design of the study; analysis of data obtained; Portnoy J.G. — analysis of data obtained, text writing; Stimmel M. — analysis of data obtained, text writing; Fo–ley F.W. — conception and design of the study; analysis of data obtained; Nehrych T.I. — conception and design of the study; analysis of data obtained.
1. Harris M.K., Maghzi A.H., Etemadifar M. et al. Acute demyelinating disorders of the central nervous system. Current Treatment Options in Neurology. 2009. 11(1). 55.
2. Voskuhl R.R. Gender issues and multiple sclerosis. Current Neurology and Neuroscience Reports. 2002. 2(3). 277–286.
3. Kessler T.M., Fowler C.J., Panicker J.N. Sexual dysfunction in multiple sclerosis. Expert Review of Neurotherapeutics. 2009. 9(3). 341–350.
4. Tepavcevic D.K., Kostic J., Basuroski I.D. et al. The impact of sexual dysfunction on the quality of life measured by MSQoL–54 in patients with multiple sclerosis. Multiple Sclerosis Journal. 2008. 14(8). 1131–1136.
5. Nortvedt M.W., Riise T., Frugård J. et al. Prevalence of bladder, bowel and sexual problems among multiple sclerosis patients two to five years after diagnosis. Multiple Sclerosis Journal. 2007. 13(1). 106–112.
6. Foley F.W., La Rocca N.G., Sanders A.S., Zemon V. Rehabilitation of intimacy and sexual dysfunction in couples with multiple sclerosis. Multiple Sclerosis Journal. 2001. 7(6). 417–421.
7. McCabe M.P., McDonald E., Deeks A.A. et al. The impact of multiple sclerosis on sexuality and relationships. Journal of Sex Research. 1996. 33(3). 241–248.
8. Vermillion S.T., Holmes M.M. Sexual dysfunction in women. Primary Care Update for OB/GYNS. 1997. 4(6). 234–240.
9. Christopherson J.M., Moore K., Foley F.W., Warren K.G. A comparison of written materials vs. materials and counselling for women with sexual dysfunction and multiple sclerosis. Journal of Clinical Nursing. 2006. 15(6). 742–750.
10. Foley F.W., La Rocca N.G., Sanders A.S., Zemon V. The multiple sclerosis intimacy and sexuality questionnaire–19 (MSISQ–19). Sexuality and Disability. 2000. 18(1). 3–26.
11. Process of translation and adaptation of instruments. World Health Organization, 2004. Available at: https://www.who.int/substance_abuse/research_tools/translation/en/
12. Polman C.H., Reingold S.C., Banwell B. et al. Diagnostic criteria for multiple sclerosis. 2010 revisions to the McDonald criteria. Annals of Neurology. 2011. 69(2). 292–302.
13. Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983. 33(11). 1444.
14. Vickrey B.G. Multiple sclerosis quality of life (MSQOL)–54 instrument. Los Angeles: University of California, 1995.
15. Nappi R.E., Cucinella L., Martella S. et al. Female sexual dysfunction (FSD). Prevalence and impact on quality of life (QoL). Maturitas. 2016. 94. 87–91.
16. Hayes R., Dennerstein L. The impact of aging on sexual function and sexual dysfunction in women. A review of population–based studies. The Journal of Sexual Medicine. 2005. 2(3). 317–330.

/33-1.jpg)
/34-1.jpg)
/35-1.jpg)