Международный неврологический журнал Том 16, №3, 2020
Вернуться к номеру
Клінічні синдроми таламічних інсультів нижньолатеральної судинної території: проспективне клінічне когортне дослідження
Авторы: S.M. Vinychuk(1), M.M. Prokopiv(2), L.M. Trepet(1), O.Ye. Fartushna(3)
(1) — Oleksandrivska Clinical Hospital, Kyiv, Ukraine
(2) — Bogomolets National Medical University, Kyiv, Ukraine
(3) — Ukrainian Military Medical Academy, Kyiv, Ukraine
Рубрики: Неврология
Разделы: Клинические исследования
Версия для печати
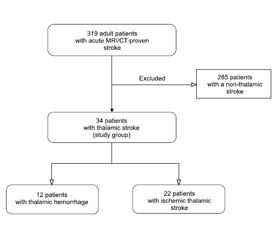
Актуальність. У статті проаналізовано i описано патофізіологічні особливості й закономірності виникнення неврологічних, нейропсихологічних та клінічних судинних синдромів таламічних інсультів нижньoлатерального судинного басейну. Мета дослідження: описати i проаналізувати клініко-нейровізуалізаційні особливості судинних синдромів таламічних інсультів нижньолатеральної судинної території в проспективному клінічному когортному дослідженні. Матеріали та методи. Ми провели проспективне клінічне когортне дослідження 319 пацієнтів iз гострим інсультом, які надійшли до неврологічного центру Олександрівської клінічної лікарні (м. Київ, Україна) протягом перших 24 годин з моменту розвитку інсульту. Усі пацієнти пройшли комплексне клініко-неврологічне, лабораторне, ультразвукове і ней-ровізуалізаційне обстеження. Результати. Серед 319 хворих iз гострим інсультом нейровізуалізаційно підтверджений таламічний інсульт діагностовано в 34 (10,6 %) пацієнтів. Iз них у 22 осіб (середній вік 61,9 ± 10,2 року) виявлено гострий ізольований інфаркт таламуса, а в 12 (середній вік 59,0 ± 9,6 року) — гострий таламічний крововилив. Проаналізовані, порівняні та описані специфічні неврологічні особливості клінічних судинних синдромів гострих таламічних інсультів нижньолатеральної судинної території.
Актуальность. В статье проанализированы и описаны патофизиологические особенности и закономерности возникновения неврологических, нейропсихологических и клинических сосудистых синдромов таламических инсультов нижнелатерального сосудистого бассейна. Цель исследования: описать и проанализировать клинико-нейровизуализационные особенности сосудистых синдромов таламических инсультов нижнелатеральной сосудистой территории в проспективном клиническом когортном исследовании. Материалы и методы. Мы провели проспективное клиническое когортное исследование 319 пациентов с острым инсультом, поступивших в неврологический центр Александровской клинической больницы (г. Киев, Украина) в течение первых 24 часов с момента развития инсульта. Все пациенты прошли комплексное клинико-неврологическое, лабораторное, ультразвуковое и нейровизуализационное обследования. Результаты. Среди 319 больных с острым инсультом нейровизуализационно подтвержденный таламический инсульт диагностирован у 34 (10,6 %) пациентов. Из них у 22 человек (средний возраст 61,9 ± 10,2 года) обнаружен острый изолированный инфаркт таламуса, а у 12 (средний возраст 59,0 ± 9,6 года) — острое таламическое кровоизлияние. Проанализированы, сравнены и описаны специфические неврологические особенности клинических сосудистых синдромов острых таламических инсультов нижнелатеральной сосудистой территории.
Background. The article covers pathophysiological features and patterns of the occurrence of neurological, neuropsychological, and clinical vascular syndromes of thalamic strokes in the lower lateral vascular territory. The purpose of this study is to determine the features of clinical vascular syndromes of an acute thalamic stroke in the lower lateral vascular territory in a prospective hospital-based cohort study, providing a comprehensive clinical and neuroimaging analysis. Materials and methods. We have prospectively recruited 319 acute stroke patients, admitted within 24 hours from the onset of the stroke symptoms to the Neurological Center at an academic hospital (Oleksandrivska Clinical Hospital) in Kyiv, Ukraine. Comprehensive neurological, clinical, laboratory, ultrasound, and neuroimaging examinations were performed to all patients. Results. MRI/CT-proven thalamic stroke was diagnosed in 34 (10.6 %) of 319 patients, forming a study group. Twenty-two of 34 patients (average age 61.9 ± 10.2 years) were diagnosed with an acute isolated ischemic thalamic infarction, and 12 people (average age 59.0 ± 9.6 years) had an acute thalamic hemorrhage. Specific neurological features of clinical vascular syndromes of acute thalamic strokes in the lower lateral vascular territory were analyzed, compared, and described.
таламус; інсульт; таламічний інсульт; клінічні особливості; синдром; судинна територія; нижньолатеральна судинна територія
таламус; инсульт; таламический инсульт; клинические особенности; синдром; сосудистая территория; нижнелатеральная сосудистая территория
thalamus; stroke; thalamic stroke; clinical features; syndrome; vascular territory; lower lateral vascular territory
Introduction
Materials and methods
Results and discussion
Conclusions
1. Benjamin E.J., Muntner P., Alonso A. [et al.]. On behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics — 2019 update: a report from the American Heart Association. Circulation. 2019. Vol. 139(10). P. e56-e528.
2. Johnson W., Onuma O., Owolabi M., Sachdev S. Stroke: a global response is needed. Bulletin of the World Health Organization. 2016. Vol. 94. P. 634-634A.
3. Vinychuk S.M., Fartushna O.Ye. Cerebrospinal and commissural diaschisis in acute stroke patients: case analysis. Meždunarodnyj nevrologičeskij žurnal. 2018. № 5(99). С. 20-25.
4. Fartushna O.Ye., Vinychuk S.M. Brain injury in patients with acute TIA: clinical features in different TIA subtypes. Meždunarodnyj nevrologičeskij žurnal. 2017. № 3(89). С. 13-18.
5. Feigin V.L., Norrving B., Mensah G.A. Global burden of stroke. Circulation research. 2017. Vol. 120(3). Р. 439-448.
6. Global Health Estimates. Geneva: World Health Organization; 2012. Режим доступу: http://www.who.int/healthinfo/global_burden_disease/en/.
7. Lees R., McGrane F., Fartushna O., Broomfield N.M., Quinn T.J., Dani K., Forbes K., Dawson J. Vascular cognitive impairment/vascular dementia. The pattern of cognitive impairment in stroke survivors with carotid stenosis. International Journal of Stroke. 2014. Vol. 9. P. 323-324.
8. Owolabi M.O., Akarolo-Anthony S., Akinyemi R. [et al.]. The burden of stroke in Africa: a glance at the present and a glimpse into the future. Cardiovasc. J. Afr. 2015. Vol. 26(2). Suppl. 1. P. S27-38.
9. Wilkins E., Wilson L., Wickramasinghe K. [et al.]. European cardiovascular disease statistics 2017. Brussels: European Heart Network, 2017. 188 p.
10. World Stroke Organization. Global Stroke Fact Sheet. 26.02.2019. Режим доступу: https://www.world-stroke.org/images/WSO_Global_Stroke_Fact_Sheet_final.pdf.
11. Віничук С.М., Прокопів М.М. Гострий ішемічний інсульт. Київ: Наукова думка, 2006. 286 с.
12. Віничук С.М., Фартушна О.Є. Рання реабілітація після гострих ішемічних порушень мозкового кровообігу. Міжнародний неврологічний журнал. 2016. № 8(86). С. 34-39.
13. Евтушенко С.К., Филимонов Д.А., Евтушенко И.С. Новые факторы риска развития инсульта у лиц молодого возраста. Журнал неврологии и психиатрии им. С.С. Корсакова. Спецвыпуск. 2015. Т. 115. № 12. С. 3-12.
14. Фартушна О.Є., Прокопів М.М. Актуальність проблеми цереброваскулярних захворювань, транзиторних ішемічних атак та вдосконалення їх діагностики в системі охорони здоров’я в Україні. Проблеми військової охорони праці: [Зб. наук. праць Української військово-медичної академії. За ред. проф. Білого В.Я.]. Київ: УВМА, 2007. Вип. 19. С. 335-342.
15. Фартушна О.Є., Віничук С.М. Виявлення та усунення васкулярних чинників ризику важливий напрямок первинної профілактики транзиторних ішемічних атак та/чи інсульту. Український медичний часопис. 2015. № 1(105). С. 23-27.
16. Фартушна О.Є., Віничук С.М. Транзиторні ішемічні атаки. Київ: ВД «Авіцена», 2014. 216 с.
17. Feigin V.L., Nguyen G., Cercy K. [et al.]. GBD 2016 Lifetime Risk of Stroke Collaborators. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N. Engl. J. Med. 2018. Vol. 379(25). P. 2429-2437.
18. Institute for Health Metrics and Evaluation (IHME). Findings from the Global Burden of Disease Study 2017. Seattle, W.A.: IHME, 2018.
19. World Stroke Organization. Facts and Figures about Stroke. Режим доступу: http://www.world-stroke.org/component/content/article/16-forpatients/84-facts-and-figures-about-stroke.
20. GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet (London, England). 2017. Vol. 390(10100). Р. 1151-1210.
21. Wang H., Naghavi M., Allen C. [et al.]. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016. Vol. 388(10053). P. 1459-1544.
22. Фартушна О.Є., Віничук С.М. Епідеміологія транзиторних ішемічних атак в структурі гострих порушень мозкового кровообігу в Україні та інших країнах. Міжнародний неврологічний журнал. 2017. № 5(91). С. 105-111.
23. Chen X.Y., Wang Q., Wang X. et al. Clinical features of thalamic stroke. Curr. Treat. Options Neurol. 2017. Vol. 19(2). P. 5.
24. Виничук С.М., Ялынская Т.А., Виничук И.С. Инфаркты в вертебробазилярном бассейне: клиника и диагностика. Междунар. неврол. журн. 2005. № 3. C. 13-21.
25. Lopez-Serna R., González-Carmona P., López-Martinez M.J. Bilateral thalamic stroke due to occlusion of the artery of Percheron in a patient with patent foramen оvale: a case report. Med. Case Reports. 2009. Vol. 3. P. 7392.
26. Bogousslavsky J., Regli F., Uske A. Thalamic infarcts: clinical syndromes, etiology, and prognosis. Neurology. 1988. Vol. 38(6). P. 837-48.
27. Cheng H., Tian Y., Hu P., Wang J., Wang K. Time-based prospective memory impairment in patients with thalamic stroke. Behavioral Neuroscience. 2010. Vol. 124(1). P. 152-158.
28. Steinke W., Sacco R.L., Mohr J.P. [et al.]. Thalamic stroke. Presentation and prognosis of infarcts and hemorrhages. Arch. Neurol. 1992. Vol. 49. P. 703-710.
29. Виничук С.М., Прокопив М.М., Трепет Л.Н. Изолированный инфаркт таламуса: клинические синдромы, диагностика, лечение и исход. Український медичний часопис. 2012. № 2. С. 87-93.
30. Виничук С.М., Прокопив М.М., Трепет Л.Н. Таламические инсульты; Нац. мед. ун-т им. А.А. Богомольца, Александр. клинич. б-ца, г. Киев, Украина. Киев: Агат-Принт, 2018. 91 с.: ил. Библиогр.: с. 82-91.
31. Vinychuk S.M., Prokopiv M.M., Trepet L.M., Fartushna O.Y. Thalamic stroke outcomes: a prospective hospital-based cohort study. Meždunarodnyj nevrologičeskij žurnal. 2019. № 8(110). Р. 23-27.
32. Kernan W.N., Ovbiagele B., Black H.R. [et al.]. Guidelines for the prevention of stroke in patients with stroke and transient ische-mic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014. Vol. 45. Р. 2160-2236.
33. Aho K., Harmsen P., Hatano S. [et al.]. Cerebrovascular di-sease in the community: results of a WHO collaborative study. Bull. World Health Organ. 1980. Vol. 58. P. 113-130.
34. Adams H.P., Bendixen B.H., Kappelle L.J. [et al.]. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993. Vol. 24. P. 35-41.
35. Ringleb P., Schellinger P.D., Hacke W. [et al.]. [European Stroke Organisation 2008 guidelines for managing acute cerebral infarction or transient ischemic attack. Part 1]. Der Nervenarzt. 2008. Vol. 79. P. 936-957.
36. Adams R., Albers G., Alberts M. [et al.]. Update to the AHA/ASA Recommendations for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack. Stroke. 2008. Vol. 39. P. 1647-1652.
37. Fartushna O.Y. Emergency therapeutic approach as a secon-dary prevention of an acute ischemic stroke in patients with TIA. XX World Neurological Congress, 12–17.11.2011. Marrakesh, Morocco, 2011. P. 167.
38. Fartushnaya E.E., Vinichuk S.M. Reducing the risk of recurrent ischemic stroke, after transient ischaemic attack along with neuroprotective and antiaggregant therapy. XIV International Congress of Rehabilitation Medicine and immunorehabilitation, 16–21.10.2009: abstract. Tel-Aviv, Israel, 2009. Р. 67.
39. Віничук С.М., Фартушна О.Є. Диференційоване лікування транзиторних ішемічних атак — ефективний спосіб профілактики повторних гострих церебральних подій. Міжнародний неврологічний журнал. 2014. № 6. С. 87-92.
40. Віничук С.М., Фартушна О.Є. Аторвастатин та його роль у профілактиці та лікуванні ішемічних порушень мозкового кровообігу. Здоров’я України. 2015. № 9. С. 3.
41. Фартушна О.Є., Віничук С.М. Використання оптимальної дози препарату Торвакард — важливий напрямок зниження ризику розвитку повторних транзиторних ішемічних атак та/чи інсульту. Семейная медицина. 2015. № 3. С. 223-227.
42. Віничук С.М., Фартушна О.Є. Рання реабілітація після гострих ішемічних порушень мозкового кровообігу. Міжнародний неврологічний журнал. 2016. № 8(86). С. 34-39.
43. Віничук С.М., Фартушна О.Є. Освітні програми профілактики транзиторних ішемічних атак та/чи інсульту. Укр. мед. часоп. 2014. № 5. С. 49-51.
44. Фартушна О.Є., Віничук С.М. Модифікація поведінкових чинників ризику як складова первинної профілактики транзиторних ішемічних атак та/чи інсульту. Український медичний часопис. 2014. № 6(104). XІ/XІІ. С. 42-44.
45. Фартушна О.Є. Патогенетичні підтипи транзиторних ішемічних атак: особливості неврологічної клініки, гемодинаміки та лікування [Текст]: Дис... канд. мед. наук: 14.01.15. Фартушна Олена Євгенівна; Нац. мед. ун-т ім. О.О. Богомольця. Київ, 2012. 217 арк.: рис., табл. Бібліогр.: арк. 187-217.
46. Vinychuk S.M., Prokopiv M.M., Trepet L.M., Fartushna O.Y. Clinical vascular syndromes of thalamic strokes in anterior and paramedian vascular territories: a prospective hospital-based cohort study. Meždunarodnyj nevrologičeskij žurnal. 2020. Vol. 2(16). P. 15-20.

/10.jpg)