Международный неврологический журнал Том 16, №3, 2020
Підвищений рівень креатинфосфокінази у хворих із віддаленими наслідками після перенесеної закритої черепно-мозкової травми
Авторы: Ye.V. Lekomtseva
State Institution “Institute of Neurology, Psychiatry and Narcology of the National Academy of Medical Sciences of Ukraine”, Kharkiv, Ukraine
Рубрики: Неврология
Разделы: Клинические исследования
Резюме
Актуальність. Формування віддалених наслідків закритої черепно-мозкової травми (ЗЧМТ) супроводжується каскадом біохімічних реакцій, які ініціюються первинною нейротравмою. Мета роботи. Ми оцінювали стан і зміни радикального окислення та маркерних мембранасоційованих ферментів, що беруть участь у різних окислювально-відновних реакціях та відображають основні метаболічні процеси організму, шляхом вимірювання рівнів креатинфосфокінази (КФК), КФК-ВВ, аденозинтрифосфату (АТФ), аденозиндифосфату (АДФ), аспартатамінотрансферази (AСT), аланінамінотрансферази (AЛT), лужної фосфатази, лактатдегідрогенази (ЛДГ) у пацієнтів із віддаленими наслідками після ЗЧМТ. Матеріали та методи. Вісімдесят два хворі (середній вік (±SD) 39,04 ± 14,62 року), тридцять здорових осіб (29,60 ± 4,73 року) були протестовані на рівень КФК, KФК-BB, ATФ, AДФ, що були вимірювані у сироватці крові спектрофотометричним методом згідно з доданими стандартними інструкціями. AЛT, AСT, лужна фосфатаза, ЛДГ були вимірювані хроматографічним та калориметричним методами. Результати. Було виявлено підвищений рівень КФК у сироватці крові хворих із віддаленими наслідками після ЗЧМТ порівняно з контролем (р < 0,001; t = 5,07), середній рівень КФК становив 163,5 ± 14,1 проти 92,6 ± 11,4 пг/мл у здорових осiб. Ми виявили дуже високий рівень KФК-BB у сироватці крові у загальній групі пацієнтів, де середній рівень KФК-BB становив 24,7 ± 2,7 проти 16,14 ± 1,83 МО/л у контрольній групі (р < 0,05; t = 4,9). Метаболічні зміни в пацієнтів полягали в більш високих показниках ЛДГ, які були прямо пропорційні збільшенню тривалості посттравматичного періоду (p < 0,05; r = +0,42); у загальній та контрольній групах середній рівень ЛДГ становив 694,1 ± 16,3 та 381,9 МО/л відповідно (р < 0,05). В обстежених хворих було виявлено дефіцит макроергічних сполук, а саме АТФ та АДФ, які знаходились у прямій залежності від тривалості захворювання, особливо понад 15 років (р < 0,05; r = +0,67); у загальній групі пацієнтів середній рівень АТФ становив 627,60 ± 12,38 мкмоль/л порівняно з 735,48 ± 14,57 мкмоль/л у групі контролю(р < 0,05); для AДФ: 256,20 ± 14,21 і 273,88 ± 11,42 (p < 0,05) відповідно. Висновки. У пацієнтів із віддаленими наслідками після ЗЧМТ були виявлені високий рівень загальної активності KФК у сироватці крові та підвищення рівня ЛДГ, що відображає біоенергетичний дисгомеостаз та тяжкість перебігу віддалених наслідків травми мозку.
Актуальность. Формирование отдаленных последствий закрытой черепно-мозговой травмы (ЗЧМТ) сопровождается каскадом биохимических реакций, которые инициируются первичной нейротравмой. Цель работы. Мы оценивали состояние и изменения радикального окисления и маркерных мембранасоциированных ферментов, которые участвуют в различных окислительно-восстановительных реакциях и отражают основные метаболические процессы организма, путем измерения уровней креатинфосфокиназы (КФК), КФК-ВВ, аденозинтрифосфата (АТФ), аденозиндифосфата (АДФ), аспартатаминотрансферазы (АСТ), аланинаминотрансферазы (АЛТ), щелочной фосфатазы, лактатдегидрогеназы (ЛДГ) у пациентов с отдаленными последствиями ЗЧМТ. Материалы и методы. Восемьдесят два больных (средний возраст (±SD) 39,04 ± 14,62 года), тридцать здоровых пациентов (29,60 ± 4,73 года) были протестированы на уровень КФК, КФК-BB, АТФ, АДФ, которые были определены в сыворотке крови спектрофотометрическим методом в соответствии с прилагаемыми стандартными инструкциями. АЛТ, АСТ, щелочная фосфатаза, ЛДГ были измерены хроматографическим и калориметрическим методами. Результаты. Был выявлен повышенный уровень КФК в сыворотке крови больных с отдаленными последствиями ЗЧМТ по сравнению с контролем (р < 0,001; t = 5,07), средний уровень КФК составлял 163,5 ± 14,1 против 92,6 ± 11,4 пг/мл у здоровых людей. Нами был выявлен очень высокий уровень КФК-BB в сыворотке крови в общей группе пациентов, у которых средний уровень КФК-BB составлял 24,7 ± 2,7 против 16,14 ± 1,83 МЕ/л в контрольной группе (р < 0,05; t = 4,9). Метаболические изменения у пациентов заключались в более высоких показателях ЛДГ, которые были прямо пропорциональны увеличению продолжительности посттравматического периода (p < 0,05; r = +0,42); в общей и контрольной группах средний уровень ЛДГ составлял 694,1 ± 16,3 и 381,9 МЕ/л соответственно (р < 0,05). У обследованных больных был выявлен дефицит макроэргических соединений, а именно АТФ и АДФ, которые находились в прямой зависимости от длительности заболевания, особенно более 15 лет (р < 0,05; r = +0,67); в общей группе пациентов средний уровень АТФ составлял 627,60 ± 12,38 мкмоль/л по сравнению с 735,48 ± 14,57 мкмоль/л в группе контроля (р < 0,05); для АДФ: 256,20 ± 14,21 против 273,88 ± 11,42 (p < 0,05) соответственно. Выводы. У пациентов с отдаленными последствиями ЗЧМТ были выявлены высокий уровень общей активности КФК в сыворотке крови и повышение уровня ЛДГ, что отражает биоэнергетический дисгомеостаз и тяжесть течения отдаленных последствий травмы мозга.
Background. The secondary traumatic brain injury (TBI) consequences are associated with multiple cascades of biochemical reactions caused by initial neurotrauma. We assessed changes in radical oxidation and marker membrane-associated enzymes involved into various redox reactions reflecting basic metabolic processes by measuring creatine phosphokinase (CPK), CPK-BB, adenosine triphosphate (ATP), adenosine diphosphate (ADP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, lactate dehydrogenase (LDH) in the patients with long-term consequences after TBI. Materials and methods. Eighty-two patients (mean age (±SD) 39.04 ± 14.62 years), thirty controls (29.60 ± 4.73 years) were tested for serum CPK, CPK-BB, ATP, ADP measured using spectrophotometry methods according to standard manufacturer’s protocols. Serum AST, ALT, alkaline phosphatase, LDH levels were detected on gas-liquid chromatograph, by calorimetric methods. Results. We found elevated serum CPK level in patients with long-term consequences after TBI compared to controls (P < 0.001, t = 5.073, 95% CI –188.6 to –92.16) with the medians of total CPK of 163.5 ± 14.1 versus 92.6 ± 11.4 pg/ml in the basic and control groups, respectively. We found abnormal high serum CPK-BB level in the basic group with medians of total CPK-BB level of 24.7 ± 2.7 versus 16.14 ± 1.83 IU/L in controls (p < 0.05; t = 4.9). Metabolic changes in our patients were associated with higher LDH content being in direct proportion with disease duration (p < 0.05; r = +0.42); the median of total LDH level was 694.1 ± 16.3 and 381.9 IU/l in basic and control groups (p < 0.05), respectively. The patients investigated were detected with deficiency of macroergic compounds: ATP and ADP being in direct proportion with disease duration over 15 years (p < 0.05; r = +0.67); the medians of total ATP level were 627.60 ± 12.38 and 735.48 ± 14.57 μmol/l (p < 0.05) in the basic and control groups; for ADP: 256.20 ± ± 14.21 versus 273.88 ± 11.42 (p < 0.05), respectively. Conclusions. Patients with long-term consequences after TBI showed higher serum CPK level associated with increased LDH level reflecting bioenergy dyshomeostasis and severity of secondary brain injuries.
Ключевые слова
креатинфосфокіназа; віддалені наслідки після закритої черепно-мозкової травми; біоенергетичний дисгомеостаз
креатинфосфокиназа; отдаленные последствия после закрытой черепно-мозговой травмы; биоэнергетический дисгомеостаз
creatine phosphokinase; long-term consequences after traumatic brain injury; bioenergy dyshomeostasis
Introduction
The secondary and delayed traumatic brain injury (TBI) consequences are associated with multiple and interacting cascades of different biochemical reactions caused by the initial neurotrauma [6, 7, 16, 18] whereas the pathogenetic mechanisms maintaining the long-term consequences after TBI are still unclear.
The previous experimental data have reported that in white or grey matter brain injuries, calcium influx is a key initiating element in molecular cascades resul-ting in both delayed cell death and/or their dysfunction, and delayed axonal disconnections [2, 4, 17, 18]. Many studies have demonstrated that in neurons calcium influx through NMDA channels results in excitotoxicity, generation of free radicals with the development of oxidative stress, mitochondrial dysfunction and postsynaptic receptor modifications [5, 6]. Other foreign reports have shown the presence of astrocytes proliferation that is typical for central nervous system injury and dysfunction resulting in a reversal of glutamate uptake and neuronal depolarization through various excitotoxic neurobiochemical mechanisms and these mechanisms depend on both energy dysfunction in neurons and primary brain injury severity [7–9, 12]. Inflammatory cells also mediate this secondary brain injury via the release of many pro-inflammatory cytokines that contribute to activation of cell-death cascades or postsynaptic receptor modifications [18, 19].
Any change in bioenergy cell homeostasis is one of the important factor determining its pathological disturbances where some key enzymes are adenosine triphosphate (ATP) that provides energy to all biochemical processes and creatine phosphokinase (CPK) that catalyzes reversible reactions of creatine and ATP to phosphocreatine or adenosine diphosphate (ADP) in muscles, brain, retina and hair cells [11, 13]. Many conditions cause changes in ATP and CPK serum levels, first of all, heart and central nervous system diseases, kidney disease, rhabdomyolysis, certain medications uptake and others [1, 3, 10].
The purpose of the study. Given the data about the development of oxidative stress and bioenergy dyshomeostasis reported in the numerous experimental and clinical studies, we assessed the changes in radical oxidation and dynamics of some membrane-associated marker enzymes involved into various redox reactions and reflecting main metabolic processes in the orga-nism by measuring complex compounds such as CPK, ATP, adenosine diphosphate, aspartate aminotransfe-rase (AST), alanine aminotransferase (ALT), alkaline phosphatase, lactate dehydrogenase (LDH) in patients with long-term consequences after traumatic brain injury.
Material and methods
Subjects. Eighty-two patients with long-term consequences after TBI (mean age (±SD) 39.04 ± 14.62 years; mean disease duration (±SD) 9.51 ± 5.70 years) and thirty age-matched healthy controls (HC; 29.60 ± ± 4.73 years) were tested for CPK, brain isoform CPK (CPK-BB), ATP, ADP, AST, ALT, alkaline phosphatase and LDH serum levels under baseline conditions. The patients were enrolled in the investigation from the Institute of Neurology, Psychiatry and Narcology from Jan 2016 to Jun 2019. The patients were aged from 18 to 73 years: 53 men (64.6 %; mean age (±SD) 46.72 ± 16.24 years; mean disease duration (±SD) 14.61 ± 9.40 years) and 29 women (35.4 %; mean age (±SD) 35.51 ± 11.03 years; mean disease duration (±SD) 6.92 ± 3.70 years). Clinical data were retrieved from the patients’ history. Exclusion criteria were craniectomy and sepsis in anamnesis, pregnancy, preexisting neurologic diseases, acute cardiovascular diseases, respiratory failure, acute or chronic liver diseases.
Methods. General activity of serum creatine phosphokinase, CPK-BB, ATP, ADP was detected by spectrophotometry methods according to standard manufacturer’s protocols using commercially available human ELISA and Dialab kits (Clinical Biochemistry; Kharkiv National Medical University). Serum AST, ALT, alkaline phosphatase, LDH levels were detected on the gas-liquid chromatograph and by calorimetric methods according to the standards providing by the manufacturer’s protocols (biochemical laboratory of the Institute of Neurology, Psychiatry and Narcology). Control and subject samples were performed in a duplicate manner. The protocols were approved by the Health Research Ethics Committee and written informed consent was obtained from each patient.
Statistical analyses. Data were analyzed according to their distribution. Age and disease duration were compared between groups with the χ2 test; parametric tests were used for normally distributed data; nonparametric tests were used for abnormally distributed data; Kruskal-Wallis and Mann-Whitney U tests were applied for the differences between groups, the multivariate analysis of covariates was performed; univariate analysis was performed to assess the relationship between various factors. All reported P values are two-tailed; P values ≤ 0.05 were considered statistically significant.
Results and discussion
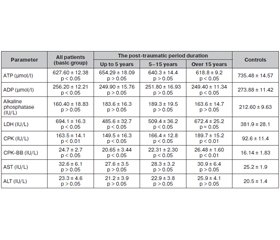
We have found the elevated creatine phosphokinase level in the serum samples of all investigated patients with long-term consequences after TBI compared to controls (Table 1, P < 0.001, t = 5.073, 95% CI –188.6 to –92.16); the medians of total creatine phosphokinase (mean ± SD) level were 163.5 ± 14.1 and 92.6 ± 11.4 pg/ml in the basic and control groups, respectively; twenty-four patients (29.26 %) had the increased serum CPK levels up to 182 % of medium CPK value (Table 1).
We found the abnormally high serum CPK-BB levels in the investigated patients when compared to controls (p < 0.05; t = 4.9); in the basic group, the median of total CPK-BB level was 24.7 ± 2.7 versus 16.14 ± 1.83 IU/L in controls (Table 1). In normal value ranges, BB-isoform serum content usually is less than 1 % and during standard diagnostic tests, we could neglect it. However, at different pathological states, serious cell and intra- or extracellular protein damage was detected following cell disturbances with consequent enzyme activity loss [1, 3, 10, 15]. We found high serum CPK-BB levels in the patients with long-term consequences after TBI that may play a certain role in the pathogenetic mechanisms of maintaining these long-term consequences.
/25.jpg)
To evaluate the clinical or prognostic value of CPK serum level changes, we further segregated among the groups with the different disease duration. When comparing the CPK content in the patients with different duration of the post-traumatic period, it was found that even the patients with the post-traumatic period less than 5 years (n = 36; 43.9 %) were characterized with up to 156.77% increase of CPK serum levels (p < 0.05) from the upper normal mean value with the median of total CPK of 149.5 ± 16.3 pg/ml. In the group of patients with the disease duration 5–15 years (n = 31; 37.8 %), the CPK content increased to 165.14 % (p < 0.05) and in the patients with the disease duration over 15 years (n = 15; 18,29 %), the CPK level elevated up to 192.97 % (p < 0.01). In our opinion, this increase of serum CPK level was a marker of increasing of membrane destruction as a result of metabolic disturbances and was a poor prognostic sign.
The standard neurological examination has revealed the presence of focal neurological signs indicating the mesencephalic and brainstem structures lesions. Among the objective focal neurological symptoms, the most frequent were face asymmetry (12 patients from group I (33.3 %); 19 patients from group II (61.29 %) and 11 patients from group III (73.3 %)), tendon reflexes increased (11 patients (30.5 %) — group I; 16 patients (51.6 %) — group II and 14 patients (93.3 %) — group III), coordination sphere disturbances (8 patients (22.2 %) — group I, 18 patients (58.06 %) — group II, 7 patients (46.6 %) — group III), ataxia (6 patients (16.6 %) — group I; 19 patients (61.29 %) — group II and all 15 patients in group III), horizontal nystagmus (5 patients (13.8 %) — group I; 14 patients (45.16 %) — group II, 9 patients (60 %) — group III), pathological foot reflexes (3 patients (8.33 %) — group I; 27 patients (87.09 %) — group II, 8 patients (53.3 %) — group III), rotatory nystagmus (8 patients (25.81 %) — group II and 6 patients (40 %) — group III), slight mono- or hemiparesis (2 patients (6.45 %) — group II, 5 patients (33.3 %) — group III).
The results of the analysis of the clinical and symptomatic manifestations in the patients with long-term consequences after TBI demonstrated that the most frequent complaint was headache (cephalgia) almost in all patients. Investigation of syndromological picture of the post-traumatic period has revealed that dizziness occurred in 44.4 % patients from group I, in 35.48 % patients from group II and in 53.33 % patients from group III. Subcortical syndromes as Marinescu-Radovici symptom and bradykinesia presented in 13.3 and 6.67 % patients from group III, respectively. The presence of shakiness was observed in 32.25 % patients from group II and 46.67 % patients from group III; visual disturbances were observed in 16.12 % patients from group II and 26.67 % patients from group III. Patients from the first (9.09 %) and second groups (12.9 %) complained of impaired sensitivity as numbness and feelings of insects crawling in the structure of nonspecific sensorimotor syndrome.
Metabolic changes in our patients appeared in higher LDH level, an important glycolysis enzyme, and this increase was in the direct proportion with the disease progression (p < 0.05; r = +0.42); the median of total LDH level was 694.1 ± 16.3 and 381.9 IU/l (p < 0.05) in the basic group and controls, respectively (Table 1). LDH is not specific only for the liver and can be elevated in many other diseases, especially accompanied by inflammation [2]. Some studies have reported that autoantibodies (abs) such as anti-nuclear abs, anti-smooth muscle abs, anti-liver and anti-kidney microsomal abs were elevated not only in the patients with autoimmune hepatitis but also in rare muscle and some neurodegenerative disorders [5, 14]. Neurological symptoms of long-term consequences after TBI can or cannot present in the patients and are associated with the mild increase of metabolic enzymes; these posttraumatic symptoms are not specific, and the pattern of membrane-associated enzyme abnormalities could provide us with the useful clarification of a cause of the condition.
Our data have shown that the patients with long-term consequences after TBI presented with baseline bioenergy dyshomeostasis appearing as a deficiency of macroergic compounds — ATP and ADP; and this decrease was in a direct proportion with the disease progression in the patient with the disease duration over 15 years (p < 0.05; r = +0.67); the median of total ATP level was 627.60 ± ± 12.38 and 735.48 ± 14.57 µmol/l (p < 0.05) in the basic group and in controls, respectively, and for ADP levels: 256.20 ± 14.21 versus 273.88 ± 11.42 (p < 0.05), respectively.
These biochemical compounds partly reflect the appearance of oxidative stress reaction contributing to the development of oxidative dyshomeostasis in this category of the patients. According to the numerous literature data, TBI results in calcium dysregulation in mitochondria [4–8, 20]; the results of our study have also confirmed the presence of oxidative stress, which have to be accounted in the treatment options. Because the patients and controls were not gender-matched, CPK and LDH levels were compared between men and women. The levels were not found to be comparable between male and female patients with long-term consequences after TBI (P = 0.196 for CPK and P = 0.272 for LDH) and controls. In the same samples, biochemical data did not correlate with the age, onset age and ATP and ADP serum levels (for CPK: P1 = 0.824 for age, P2 = 0.673 for onset age, P3 = 0.54 for ATP and P4 = 0.59 for ADP and for LDH: P1 = 0.72, P2 = 0.591, P3 = 0.43; P4 = 0.57, respectively). Our results showed no significant relationships between LDH and CPK levels and bioenergy dyshomeostasis as in patients the serum ATP levels remained low during this time.
Thus, in this study, the effect of TBI oxidative stress parameters was assessed where a generation of reactive oxygen species in the pathogenesis of maintaining of long-term consequences after traumatic brain injury plays a definite role in neuronal and neurological dysfunction and where CPK levels may serve as a biochemical marker of severity of long-term consequences after TBI. Some authors have shown that possible mechanism for releasing of CPK was transient membrane rupture after trauma or inflammation; also, some experimental studies showed that another way to CPK release might be the possible cell necrosis leading to CPK release [3, 4, 6, 15].
Long-term consequences after TBI is a pathological condition leading to progressive neuronal dysfunction where changes of some marker membrane-associated enzymes and various biochemical compounds of the oxidative status partly reflects the development of oxidative stress associated with bioenergy dyshomeostasis. Our study describes the detection of CPK as a brain biomarker to determine the severity of the secondary brain injury in patients with long-term consequences after TBI.
Conclusions
The patients with long-term consequences after TBI showed the abnormal high serum creatine phosphokinase levels associated with increased LDH levels reflecting the bioenergy dyshomeostasis and severity of the secondary brain injuries.
Conflicts of interests. Author declares the absence of any conflicts of interests and his own financial interest that might be construed to influence the results or interpretation of their manuscript.
Acknowledgement. Yevgeniya Lekomtseva, MD, PhD, Associate Professor, would like to greatly thank Gorbatch T.V., PhD (Kharkiv National Medical University, Department of Biochemistry) for her great biochemical support in this research.
Data on financial support. This research did not receive any grant support.
Список литературы
1. Aljuani F., Tournadre A., Cecchetti S., Soubrier M., Dubost J.J. Macro-creatine kinase: a neglected cause of elevated creatine kinase. Intern. Med. J. 2014. 5 (4). 457-459.
2. Bazan N.G. Second messengers derived from excitable membranes are involved in ischemic and seizure-related brain damage. Path. Physiol. and Exper. Therapy. 1999. 4. 11-16.
3. Bazarian J.J., Beck C., Blyth B., von Ahsen N., Hasselblatt M. Impact of creatine kinase correction on the predictive value of S-100B after mild traumatic brain injury. Restor. Neurol. Neurosci. 2006. 24 (3). 163-172.
4. Czigler A., Toth L., Szarka N., Berta G., Amrein K. et al. Hypertension exacerbates cerebrovascular oxidative stress induced by mild traumatic brain injury: protective effects of the mitochondria-targeted antioxidative peptide SS-31. J. Neurotrauma. 2019. 36 (23).
5. Halliwell B., Gutteridge J.M. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet. 2000. 1 (8391). 1396-1398.
6. Harmon J.L., Gibbs W.S., Whitaker R.M., Schnellmann R.G., Adkins D.L. Striatal mitochondrial disruption following severe traumatic brain injury. J. Neurotrauma. 2017. 34 (2). 487-494.
7. Hill R.L., Singh I.N., Wang J.A., Hall E.D. Time courses of post-injury mitochondrial oxidative damage and respiratory dysfunction and neuronal cytoskeletal degradation in a rat model of focal traumatic brain injury. Neurochem. Int. 2017. 111. 45-56.
8. Hubbard W.B., Joseph B., Spry M., Vekaria H.J., Saatman K.E., Sullivan P.G. Acute mitochondrial impairment underlies prolonged cellular dysfunction after repeated mild traumatic brain injuries. J. Neurotrauma. 2019. 36 (8). 1252-1263.
9. Jannas-Vela S., Brownell S., Petrick H.L., Heigenhauser G.J.F., Spriet L.L., Holloway G.P. Assessment of Na+/K+ ATPase ativity in small rodent and human skeletal muscle samples. Med. Sci. Sports Exerc. 2019. 51 (11). 2403-2409.
10. Kilianski J., Peeters S., Debad J., Mohmed J. et al. Plasma creatine kinase B correlates with injury severity and symptoms in professional boxers. J. Clin. Neurosci. 2017. 45. 100-104.
11. Kotwal G.J., Sarojini H., Chien S. Pivotal role of ATP in macrophages fast tracking wound repair and regeneration. Wound Repair. Regen. 2015. 23 (5). 724-727.
12. Lekomtseva Y., Voloshyn-Gaponov I., Gorbach T. Targeting higher levels of tau protein in Ukrainian patients with Wilson’s disease. Neurol. Ther. 2019. 8 (1). 59-68.
13. Li X., Wang H., Wen G., Li L., Gao Y., Zhuang Z., Zhou M., Mao L., Fan Y. Neuroprotection by quercetin via mitochondrial function adaptation in traumatic brain injury: PGC-1α pathway as a potential mechanism. J. Cell. Mol. Med. 2018. 22 (2). 883-891.
14. Martin L.J., Wong M., Hanaford A. Neonatal brain injury and genetic causes of adult-onset neurodegenerative disease in mice interact with effects on acute and late outcomes. Front. Neurol. 2019. 18 (10). 635.
15. McLeish M.J., Kenyon G.L. Relating structure to mechanism in creatine kinase. Crit. Rev. Biochem. Mol. Biol. 2005. 40 (1). 1-20.
16. Morris M.C., Bercz A., Niziolek G.M., Kassam F. et al. UCH-L1 is a poor serum biomarker of murine traumatic brain injury after polytrauma. J. Surg. Res. 2019. 3 (244). 63-68.
17. Papa L., Ramia M.M., Edwards D., Johnson B.D., Slobounov S.M. Systematic review of clinical studies examining biomarkers of brain injury in athletes after sports-related concussion. J. Neurotrauma. 2015. 32 (10). 661-673.
18. Park E., Bell J.D., Baker A. Traumatic brain injury: Can the consequences be stopped? CMAJ. 2008. 178 (9). 1163-1170.
19. Phillips L.L., Lyeth B.G., Hamm R.J., Povlishock J.T. Combined fluid percussion brain injury and entorhinal cortical lesion: a model for assessing the interaction between neuroexcitation and deafferentation. J. Neurotrauma. 1994. 11 (6). 641-56.
20. Phillips L.L., Reeves T.M. Interactive pathology following traumatic brain injury modifies hippocampal plasticity. Restor. Neurol. Neurosci. 2001. 19 (3–4). 213-35.

/25.jpg)