Резюме
Актуальність. У статті проаналізований характер змін фенотипового складу лімфоцитів крові, вмісту IgA, IgG, IgM і фракційного складу імунних комплексів у пацієнтів з ішемічним інсультом і хронічною ішемією мозку. Мета дослідження: вивчити характер порушень клітинної і гуморальної ланок імунітету в гострому періоді ішемічного інсульту. Матеріали та методи. Провели порівняльне клініко-імунологічне обстеження 85 пацієнтів у гострому періоді ішемічного інсульту (основна група) та 66 пацієнтів із хронічною ішемією мозку (контрольна група). Для оцінки клітинного імунітету використовували показники імунофенотипування клітин крові — абсолютний і відсотковий вміст популяції Т- і В-лімфоцитів, їх основних субпопуляцій: Т-хелперів, цитотоксичних Т-клітин, субпопуляції натуральних кілерів. Для оцінки гуморального імунітету досліджували вміст основних класів імуноглобулінів (IgA, IgG, IgM) і циркулюючих імунних комплексів. Результати. У гострому періоді ішемічного інсульту спостерігали підвищення кількості лейкоцитів (р = 0,028) і зниження рівня лімфоцитів (р = 0,031), зрілих Т-лімфоцитів (р = 0,002), Т-хелперів (р = 0,027) і цитотоксичних Т-лімфоцитів (р = 0,045). Відзначена тенденція до зниження кількості натуральних кілерів (р = 0,45). У гуморальній ланці імунітету спостерігали підвищення В-лімфоцитів (CD20+) (р = 0,048), дисгаммаглобулінемію за рахунок підвищення рівня IgG (р = 0,048) і тенденції до гіперфункції IgА і IgМ (р = 0,05). Зміни показників імунного статусу більш виражені при зростанні тяжкості інсульту. Висновки. Це дослідження доводить залучення імунної системи до складного комплексу реакцій, які беруть участь у розвитку інфарктів мозку.
Актуальность. В статье проанализирован характер изменения фенотипического состава лимфоцитов крови, содержания IgA, IgG, IgM и фракционного состава иммунных комплексов у пациентов с ишемическим инсультом и хронической ишемией мозга. Цель исследования: изучить характер нарушений клеточного и гуморального звеньев иммунитета в остром периоде ишемического инсульта. Материалы и методы. Провели сравнительное клинико-иммунологическое обследование 85 пациентов в остром периоде ишемического инсульта (основная группа) и 66 пациентов с хронической ишемией мозга (контрольная группа). Для оценки клеточного иммунитета использовали показатели иммунофенотипирования клеток крови — абсолютное и процентное содержание популяции Т- и В-лимфоцитов, их основных субпопуляций: Т-хелперов, цитотоксических Т-клеток, субпопуляции натуральных киллеров. Для оценки гуморального иммунитета исследовали содержание основных классов иммуноглобулинов (IgA, IgG, IgM) и циркулирующих иммунных комплексов. Результаты. В остром периоде инсульта наблюдали повышение количества лейкоцитов (р = 0,028) и снижение уровня лимфоцитов (р = 0,031), зрелых Т-лимфоцитов (р = 0,002), Т-хелперов (р = 0,027) и цитотоксических Т-лимфоцитов (р = 0,045). Отмечена тенденция к снижению количества натуральных киллеров (р = 0,45). В гуморальном звене иммунитета наблюдалось увеличение В-лимфоцитов (р = 0,048), дисгаммаглобулинемия за счет повышения уровня IgG (р = 0,048) и тенденции к гиперфункции IgА и IgМ (р = 0,5). Изменения показателей иммунного статуса более выражены при увеличении тяжести инсульта. Выводы. Данное исследование доказывает вовлечение иммунной системы в сложный комплекс реакций, участвующих в развитии инфарктов мозга.
Background. The article analyzes the nature of the change in the phenotypic composition of blood lymphocytes, the content of IgA, IgG, IgM, and the fractional composition of immune complexes in patients with ischemic stroke and chronic brain ischemia. The purpose of this study was to investigate the nature of disorders of cellular and humoral parts of the immune system in the acute period of ischemic stroke. Materials and methods. A comparative clinical and immunological examination was performed in 85 patients in the acute period of ischemic stroke (basic group) and 66 patients with chronic cerebral ischemia (control group). To assess cellular immunity, we used the indicators of immunophenotyping of blood cells — the absolute and percentage content of the population of T- and B-lymphocytes, their main subpopulations: T-helper cells, cytotoxic T-cells, subpopulations of natural killers. To evaluate humoral immunity, the contents of the main classes of immunoglobulins (IgA, IgG, IgM) and circulating immune complexes were studied. Results. In the acute period of stroke, an increase in the number of leukocytes (p = 0.028) and a decrease in lymphocytes (p = 0.031), T-lymphocytes (p = 0.002), T-helpers (p = 0.027) and cytotoxic T-lymphocytes (p = 0.045) were observed. There was also a tendency to a decrease in the number of natural killers (p = 0.45). In the humoral immunity an increase in the B-lymphocytes (p = 0.048), dysgammaglobulinaemia due to an increase in IgG level (p = 0.048) and the tendency to hyperfunction of IgA and IgМ (p = 0.05) were observed. Changes in immune status indicators are more pronounced with increasing severity of stroke. Conclusions. These studies demonstrate the involvement of the immune system in a complex set of reactions involved in the development of cerebral accidents.
Introduction
Acute cerebrovascular disorders continue to dominate in the structure of vascular diseases of the brain [1]. Stu-
dies in recent years have shown the complexity and versatility of the pathogenesis of ischemic stroke. Some authors have summarized the data of experimental and clinical studies, which allowed to formulate the concept accor-ding to which the development of cerebral ischemia is accompanied by a complex response of the neuro-immuno-endocrine system [2–7].
The interaction of the nervous and immune systems, carried out on the principle of mutual regulation, determines the risk of dysfunction of one of them in the pathology of the other [8–10]. Recently, in the pathogenesis of ischemic stroke great importance is given to immunological mechanisms, including the autoimmune process, which worsens the clinical picture and contributes to the development of neurological deficits. The antibodies to DNA in the acute period of ischemic stroke form as a result of intense destructive processes in the brain, which are accompanied by cell decay and disruption of homeostatic tissue processes. Moreover, these indicators correlate with the severity of the pathological process and the degree of neurological deficit regression — the higher the level of antibodies to DNA, the more pronounced the neurological deficit [11]. The main mechanism in the pathogenesis of stroke is damage to the endothelium of the vascular wall, which occurs with the participation of immune factors and is associated with the deposition of immune complexes on the inner surface of blood vessels [12].
Analysis of the literature on the parameters of immune status in cerebrovascular pathology revealed that its deve-lopment is accompanied by leukocytosis in combination with relative lymphopenia, suppression of T-cell immune system (reduction of mature CD3+, immunoregulatory CD4+, the cytotoxic activity of CD8+ T-lymphocytes) with an increase in the content of B-lymphocytes (CD19+, CD20+), IgA, IgG, IgM, and circulating immune complexes (CIC) [13, 14].
In a very severe stroke, a more pronounced degree of lymphopenia, decreased T-cell immunity (T-lymphocytes, T-helpers) [15] and activation of the humoral response (increased levels of IgA and CIC) were noted. Immune status indicators correlated with the functional result: the more severe the degree of disability (3–4 degree on the Rankin scale), the lower the levels of T-lymphocytes, T-helpers, IgM and higher IgA [13]. In a study by A. Hug et al. [16], a relationship was found between immunological parameters and specific characteristics of stroke, such as the assessment of neurological deficit on the National Institutes of Health Stroke Scale (NIHSS) and the volume of the heart attack.
The main factor determining the development of lymphocytopenia, mainly due to natural killer cells, on the 1st and 4th days after stroke, is the volume of infarction, which was an independent early predictor of the development of respiratory infections. However, another study found no statistically significant association between infarct focal volume and post-stroke T-cell count [17].
Thus, despite a large number of studies on this problem, to date, there is insufficient clinical data along with a lack of a comprehensive assessment of cellular and humoral immune factors in patients in the acute period of ischemic stroke. Little data has been published to support the role of adhesion molecules in the development of the immune response and the processes associated with endothelial dysfunction in cerebral ischemia. Methods for determining the factors of intercellular interaction remain inaccessible due to the high complexity of their implementation and the high cost of test systems.
The subject of our study was focused on the influence of pathogenetic factors of cellular and humoral immunity on the course of the acute period of stroke.
The purpose of this study was to investigate the nature of disorders of cellular and humoral parts of the immune system in the acute period of ischemic stroke.
Materials and methods
The clinical and immunological examination was performed in 85 patients (71 men and 14 women) in the acute period of ischemic stroke (basic group), who were treated in the neurological intensive and angio-neurological departments of the Military Medical Clinical Center of the Western region (Lviv). The age of the patients ranged from 31 to 87 years (mean age 61.4 ± 8.2 years). Thirty-one (36.5 %) patients were diagnosed with a stroke of the left middle cerebral artery, 30 (35.3 %) — of the right middle cerebral artery, and 24 (28.2 %) — of the vertebrobasilar system.
The control group consisted of 66 patients (52 men and 14 women) with chronic cerebral ischemia. The mean age of patients in the control group was 61.6 ± 8.5 years.
Clinical diagnoses of ischemic stroke and chronic cerebral ischemia were established based on the anamnestic data, assessment of subjective and objective neurological symptoms and the results of additional methods of examination (duplex scanning of the main arteries of the head, computed tomography of the brain) according to ICD-10. The severity of neurological symptoms was assessed on the –NIHSS and was 7.9 ± 0.7 points.
Criteria for the exclusion of patients from the study were as follows: subarachnoid hemorrhage, brain hematoma, other (non-vascular) diseases of the central nervous system, cancer, severe coronary heart disease, acute myocardial infarction, chronic pulmonary, renal or hepatic failure, HIV infection.
The indicators of immunophenotyping of blood cells were used to assess cellular immunity — the absolute and percentage of the population of T- and B-lymphocytes, their main subpopulations: T-helpers, cytotoxic T-cells, subpopulations of natural killers (NK-cells). Phenotyping of peripheral blood lymphocytes was performed by indirect immunofluorescence using monoclonal antibodies to clusters of differentiation CD3+, CD4+, CD8+, CD16+, CD20+. The FITC fluorescent label (fluorescent isothiocyanate) was used. To detect an imbalance in the composition of immunoregulatory subpopulations, we determined the immunoregulatory index (IRI), which is the ratio of the percentage content of subpopulations of phenotypes CD4+/CD8+. The indicators of the phenotypic composition of lymphocytes of patients were compared with the data of their average content in the blood of 20 relatively healthy individuals.
To assess humoral immunity, the serum content of the main classes of immunoglobulins (IgA, IgG, IgM) was detected by radial immunodiffusion (Manchini G., 1965) using monospecific antisera. The determination of the CIC is based on the possibility of sequential deposition of the fractional composition of large, medium and small complexes using increasing concentrations of 2%, 3.75%, 5.5% solutions of polyethylene glycol with a molecular weight of 6000 (in borate buffer, pH = 8.4). The amount of depo-sited CIC was determined by the difference in optical density of the forming precipitate measured on a spectrophotometer creatine phosphokinase type 3-01 at a wavelength of L = 450 nm.
The study used applicable packages of Statistica for Windows v. 8.0 (StatSoft Inc., USA, 2012) in accordance with the recommendations for processing the results of biomedical research.
Results and discussion
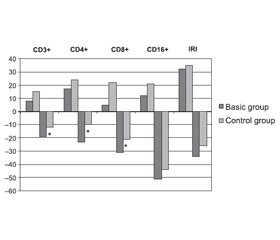
In the basic group, the changes in the phenotypic composition of lymphocytes were characterized by heterogeneity and severity, showing signs of immune deficiency with the development of general lymphopenia. The number of patients with CD3+, CD4+ and CD8+ deficiency significantly increased (Fig. 1).
The control group most often showed a decrease in the relative and absolute number of natural killers (CD16+), multidirectional changes in the content of cytotoxic CD8+ cells and the value of IRI. The average amount of CD16+ in the control group was generally at the lower limit of normal (Fig. 1).
In the basic group, the number of patients with reduced rates of certain subpopulations of immunocompetent cells increased significantly. The multidirectional nature of changes in the total content of leukocytes and the percentage of lymphocytes in the blood of patients with ischemic stroke should be noted, which is a significant decrease in the relative content of blood lymphocytes against the background of increasing total content of leukocytes. In this case, "true" leukocytosis in the basic group developed in particular cases. This type of change in the blood content of leukocytes and lymphocytes in the acute period of ischemic stroke was the most common and characteristic.
The changes in the phenotypic composition of lymphocytes were generally supplemented by a significant decrease in the relative level of mature T-lymphocytes (CD3+) (p = 0.002) and subpopulation composition of T-lymphocytes, which was characterized by a significant decrease in relative and absolute T-helpers (CD4+) (p = 0.027) and cytotoxic T-lymphocytes (CD8+) (p = 0.045). Significant differences in the IRI were not obtained (p = 0.11). There is also a tendency to the reduction of the content of natural killers (CD16+).
The changes in humoral immunity in the basic group, in addition to a significant increase in the number of B-lymphocytes (CD20+) (p = 0.048) and IgG (p = 0.048), were represented by a wide range of divergent deviations of IgA and IgM without significant differences in their mean level compared to the control group. However, in 21 (24.7 %) patients of the basic group, the level of IgG was reduced which can be regarded as a manifestation of immunodeficiency of the humoral immune system.
Thus, the acute period of ischemic stroke is characte-rized by dysregulation of the cellular immune system with an imbalance of subpopulations of CD4+ and CD8+ cells, multidirectional changes in IRI and CD16+ cell content. The general picture of changes in immune status in patients of the basic group is represented by leukocytosis with lymphopenia, a combination of signs of dysregulation and immunodeficiency of cellular and humoral immune systems, which can lead to post-stroke complications associated with both immune deficiency and autoimmune disorders.
The deficiency of the T-cell-mediated immunity may be due to the accumulation of lymphocytes in the lymphoid organs and the delay in the release of their precursors against the background of cerebral ischemia. The decrease in mature T-lymphocytes (CD3+), immunoregulatory subpopulations of T-helpers (CD4+) and cytotoxic T-lymphocytes (CD8+) can also be explained by their penetration through the disrupted blood-brain barrier into the ischemic locus and participation in the local immune system.
The acute period of ischemic stroke is characterized by a variety of relative and absolute contents of T-cells, CD4+ subpopulations, and, to a lesser extent, cytotoxic suppressors (CD8+). Besides, in the acute period of ischemic stroke, there was a decrease in IRI. This was due to a decrease in the percentage of CD4+ cells with relative preservation of CD8 + cells. In the basic group, a significant increase in all CIC fractions was found (Table 1).
In the majority of patients in the control group, the revealed changes of the indicators of a cellular link of immunity are represented by the imbalance of subpopulations of CD4+ and CD8+ cells and changes of IRI, more often in the form of its increase. In combination with CD16+ cell deficiency, the increased expression of activation markers, and IgM levels likely associated with polyclonal activation of humoral immunity, the discovered changes may reflect the development of an autoimmune process.
When comparing the immune status of patients with varying degrees of stroke severity (Table 2), it was noted that the rates of lymphopenia (p = 0.022) and decreased content of T-lymphocytes (CD3+) (p = 0.033) were significantly lower in moderate and severe stroke compared to patients with the mild course. There were significant differences in subpopulations of T-lymphocytes (CD4+) (p = 0.034) and (CD8+) (absolute values) (p = 0.046), which correlated with the severity of stroke. In severe ischemic stroke, a more pronounced decrease in NK-cells (CD16+) (p = 0.16) developed. The analysis of humoral immunity demonstrated a significant difference in the number of B-lymphocytes (p = 0.047), IgG (p = 0.048), and all fractions of the CIC in patients with moderate and severe stroke.
/21.jpg)
Decreased levels of T-lymphocytes (CD3+), T-helpers (CD4+), T-cytotoxic lymphocytes (CD8+), NK-cells (CD16+) are an indirect sign of the severity of the ischemic stroke, the risk of complications and the possibile unfavorable prognosis of ischemic stroke.
Thus, the study made it possible to identify and investigate the nature of changes in the cellular part of the immune system and some indicators of humoral immunity in patients in the acute period of ischemic stroke, as well as with chronic cerebral ischemia. The heterogeneous nature of these changes in patients with chronic cerebral ischemia, as well as the higher severity and features of immune status in patients with acute ischemic stroke are described. The detected changes in the immune system in patients in the acute period of ischemic stroke are an example of neuroimmune interaction in response to acute stress.
Conclusions
1. Changes in immune status were more pronounced in the acute period of ischemic stroke compared to patients with chronic cerebral ischemia and depended on the stroke severity.
2. The general picture of changes in immune status in patients in the acute period of ischemic stroke is represented by a combination of signs of dysregulation and immunodeficiency of cellular and humoral parts of immunity, leukocytosis with lymphopenia.
3. The picture of the changes in the phenotypic composition of lymphocytes in patients with ischemic stroke is characterized by a deficiency of populations of CD3+, CD4+, CD8+ cells. Changes in humoral immunity are represented by an increase in the number of B-lymphocytes (CD20+), IgG, and an increase in all fractions of the CIC.
4. This study proves the involvement of the immune system in a complex set of reactions involved in the development of cerebral infarction. Therefore, the assessment of the parameters of the immune system in such patients is of great practical importance for the treatment. If disturbances of the basic parameters of the immune system are detected, patients with ischemic stroke should be examined by an immunologist for appropriate immuno-correction.
Conflicts of interests. Authors declare the absence of any conflicts of interests and their own financial interest that might be construed to influence the results or interpretation of their manuscript.
Author contributions: S.M. Stadnik — study concept and design, statistical analysis, interpretation of data, literature overview, critical revision of the manuscript for important intellectual content; O.V. Saiko — data acquisition, statistical analysis, critical revision of the manuscript for important intellectual content; I.V. Hayda — translation of the article into English, critical revision of the manuscript for important intellectual content.
Список литературы
1. Инсульт: диагностика, лечение, профилактика. Под ред. Суслиной З.А., Пирадова М.А. М.: МЕДпресс-информ, 2008. 288 с.
2. Байракова А.Л., Воропаева Е.А., Афанасьев С.С. и др. Роль и биологическое значение ТОЛЛ-подобных рецепторов в антиинфекционной резистентности организма. Вестник Российской АМН. 2008. № 1. С. 45-54.
3. Владимиров В.Г., Васильева И.Н., Шарова Л.А. Внеклеточная ДНК и ее значение для современной медицины. Клиническая медицина и патофизиология. 1998. № 1–2. С. 110-119.
4. Ганнушкина И.В. Аспекты дизрегуляции в патогенезе нарушений мозгового кровообращения. М.: Медицина, 2002. С. 260-293.
5. Ганнушкина И.В., Антелава А.Л., Вейко Н.Н. Гидродинамическая (негенетическая) эффективность разных форм ДНК. Патологическая физиология и экспериментальная терапия. 2000. № 4. С. 3-5.
6. Гусев Е.И. Основные механизмы острой церебральной ишемии. Мозг: теоретические и клинические аспекты. М.: Медицина, 2003. С. 139-157.
7. Рабсон А., Ройт А., Делвз П. Основы медицинской иммунологии. Пер. с англ. М.: Изд-во «Мир», 2006. 315 с.
8. Никифорова Т.А., Песков С.А., Доронина О.Б. Анализ современного состояния клинико-экспериментальных данных о взаимодействии нервной и иммунной систем. Бюллетень сибирской медицины. 2014. Т.13. № 6. С.72-80. doi: 10.20538/1682-0363-2014-6-72-80
9. Созаева Д.И., Бережанская С.Б. Основные механизмы взаимодействия нервной и иммунной систем. Клинико-экспериментальные данные. Кубанский научный медицинский вестник. 2014. № 3 (145). С.145-150.
10. Cruz Y., Cantú-Saldaña K., Ibarra А. Immune System Involvement in the Degeneration, Neuroprotection, and Restoration after Stroke. Ischemic Stroke-Updates. 2016. doi: 10.5772/64318. Available at: www.intechopen.com/books/ischemic-stroke-updates/immune-system-involvement-in-the-degenerationneuroprotection-and-restoration-after-stroke
11. Жданов Г.Н., Герасимова М.М. Роль антител к ДНК в прогнозировании течения ишемического инсульта. Юбилейная X конференция «Нейроиммунология»: сб. мат-лов. 2001. Т. 2. 49 с.
12. Бакунц Г.О. Эндогенные факторы церебрального инсульта. М.: ГЭОТАР-Медиа, 2011. 360 с.
13. Кашаева Л.Н., Карзакова Л.М., Саперов В.Н. Иммунологические нарушения при церебральных инсультах и их коррекция. Медицинская иммунология. 2005. Т. 7. № 1. С. 57-62. doi: 10.15789/1563-0625-2005-1-57-62
14. Охтова Ф.Р. Ишемический инсульт и показатели клеточного и гуморального иммунитета (клинико-иммунологическое исследование): автореф. дис. … канд. мед. наук. М., 2014. 29 с.
15. Ребенко Н.М. Клинико-иммунологические особенности у больных в остром периоде ишемического инсульта: автореф. дис. … канд. мед. наук. Новосибирск, 2004. 24 с. Режим доступа: https://static.freereferats.ru/_avtoreferats/01004298611.pdf
16. Hug A., Dalpke A., Wieczorek N., Giese T., Lorenz A., Auffarth G., Liesz A., Veltkamp R. Infarct volume is a major determiner of post-stroke immune cell function and susceptibility to infection. Stroke. 2009. Vol. 40, № 10. P. 3226-3232. Available at: www.ahajournals.org/doi/full/10.1161/STROKEAHA.109.557967
17. Haeusler K.G., Schmidt W.U., Fohring F., Meisel C., Helms T., Jungehulsing G.J., Nolte C.H., Schmolke K., Wegner B., Meisel A., Dirnagl U., Villringer A., Volk H.D. Cellular immunodepression preceding infectious complications after acute ische-mic stroke in humans. Cerebrovasc. Dis. 2008. Vol. 25, № 1–2. Р. 50-58. Available at: www.karger.com/Article/Abstract/111499

/20.jpg)
/21.jpg)