Introduction
Disorders of higher cerebral functions are one of the most pressing medical and social problems, as they lead to a decrease in the quality of life, disorders of social and professional activities, and in the long run — to the development of dementia and complete social disadaptation [1–4]. Early diagnosis of cognitive disorders makes it possible to prescribe timely treatment and predict the onset of disability. Predementia cognitive disorders are of clinical significance since they are more amenable to therapeutic correction [5]. Detection of early, potentially reversible cognitive disorders on the background of cardiovascular pathology gives the opportunity to quickly identify groups of patients with an increased risk of cognitive dysfunction, especially among working-age population.
Most studies on cognitive disorders have investigated the role of arterial hypertension and cerebral atherosclerosis in their occurrence [6–9] but the effect of cardiac arrhythmias on the development of cognitive deficits has not been sufficiently studied.
One of the risk factors for cognitive decline is a genetic predisposition caused by a mutation in the apolipoprotein E (APOE, ε4) gene on chromosome 19 [10–12]. Carrying the ε4 allele of the APOE gene is associated with an increased risk of developing Alzheimer’s disease, as well as an earlier age of cognitive disorders. The relationship between APOE4 and β-amyloid causes the formation of atherosclerotic plaques, and the relationship with the tau protein explains the formation of neurofibrillary tangles [12–14].
Various studies, including those in non-dementia patients, have shown the effect of APOE4 on memory, information processing, and other aspects of cognitive functions [15–17]. Identification of genetic factors associated with a high risk of cognitive dysfunction would make it possible to take preventive measures long before the onset of clinical symptoms.
An integrated approach to the study of the mechanisms of cognitive disorders in patients with arrhythmias, their early diagnosis, choosing the right treatment strategy for arrhythmias can slow the progression of cognitive deficits, which will improve not only the clinical status of patients but also their prognosis. The above positions determined the relevance of the chosen direction of research and its purpose.
The purpose of this study was to investigate the relationship between genetic (APOE gene polymorphism) indicators and the development of cognitive disorders in patients with arrhythmias.
Materials and methods
One hundred and ten patients aged from 30 to 75 years (mean age 63.8 ± 4.3 years old) were examined, of which 86 individuals were in the basic group and 24 in the control group. The basic group included patients with cognitive disorders on the background of different forms of arrhythmias: 31 (36 %) with persistent (paroxysmal) form of atrial fibrillation, 26 (30.2 %) with permanent form of atrial fibrillation, 16 (18.6 %) with atrioventricular block degrees ІI–III, and 13 (15.2 %) people with sick sinus syndrome. The control group was represented by patients with arrhythmias without cognitive disorders.
Background diseases, which led to the development of arrhythmias, were ischemic and/or hypertensive heart dise-ase — 58 (67.4 %) cases, non-coronary myocardial diseases — 14 (16.3 %), chronic rheumatic heart disease — 8 (9.3 %), thyroid pathology — 6 (7 %).
Criteria for the inclusion of patients in the study: age up to 75 years; the presence of arrhythmias verified on the basis of clinical data, electrocardiography (ECG); daily ECG monitoring; patient’s ability to carry out productive contact with a doctor to evaluate cognitive status; voluntary informed patient’s consent or, if necessary, the consent of the person who takes care of the patient.
Criteria for the exclusion of patients in the study: the absence of a voluntary informed patient’s consent; vascular dementia (total score on the Mini-Mental State Examination (MMSE) < 24, on the Frontal Assessment Battery (FAB) < 11, on the Mattis Dementia Rating Scale (MDRS) < 115); other possible causes of cognitive disorders, including cerebrovascular diseases: Parkinson’s disease and parkinsonian syndrome, Huntington’s disease, Wilson-Konovalov disease, normal pressure hydrocephalus, brain tumors (primary and metastatic), neuroinfections, epilepsy, demyelinating di-seases, Alzheimer’s disease, frontotemporal degeneration, Lewy body dementia; brain injuries and their consequences, which are the only cause of cognitive deficits; acute cerebrovascular disorders; unstable angina, myocardial infarction during the last 3 months; any somatic diseases in the stage of decompensation, mental illness or alcoholism (including daily consumption of more than 30 ml of alcohol for the last 3 months), drug dependence. These criteria are due to the need to separate as much as possible the influence on the study results of pathology with a proven effect on cognitive functions.
Neuropsychological testing [18–20] was performed to rule out dementia. It included screening with MMSE (M. Folstein et al., 1975), FAB (B. Dubois et al., 2000), MDRS (S. Mattis, 1976), State-Trait Anxiety Inventory (C.D. Spilberger et al., 1976), Beck’s Depression Inventory (A.T. Beck et al., 1975). Extensive neuropsychological testing was performed to determine the neuropsychological profile and the structure of cognitive disorders [21–25]. It included the following tests: 10 words (A.R. Luria, 1969), 5 words (E. Grober et al., 1988), verbal association test (A. Kazdin, 1982), Judgment of Line Orientation (A. Benton, 1975), unpainted objects (A.R. Luria, 1969), clock drawing (T. Sunderland et al., 1989), test of connection of numbers and letters (Trail Making Test) (R.M. Reitan, 1958), Boston Naming Test (J. Kaplan et al., 1978). Mild cognitive disorders were determined by the criteria of N.N. Yakhno et al. (2005), moderate cognitive disorders — by the criteria of R.S. Petersen (2004).
A comparative analysis of the frequency of genotypes and alleles of polymorphic variants of the APOE gene was conducted. The study consisted of two stages. In the first stage, DNA was isolated from the nuclei of peripheral leukocytes. In the second stage, a polymerase chain reaction of the APOE gene fragment was performed, followed by restriction product length analysis with 6% polyacrylamide gel electrophoresis. The results of gel electrophoresis were studied by viewing and photographing on the Praktika device (Germany) in transmitted ultraviolet light on the MacroVue transilluminator (LKB, UK).
Based on the results, the presence of the ε2, ε3 or ε4 allele and the possible genotype of the patient (ε2/ε2, ε2/ε3, ε3/ε3, ε2/ε4, ε3/ε4, ε4/ε4) were determined. The choice of patient groups was determined by the widely available literature on the influence of the APOE genotype not only on the state of cognitive functions but also on vascular risk factors.
The results of statistical processing of quantitative variables are represented by the means and standard deviations (M ± SD). In the study, application package Statistica for Windows v. 8.0 (StatSoft Inc, USA, 2012) was used in accordance with the recommendations for processing the results of biomedical research.
Results and discussion
With an objective evaluation of the cognitive sphere in the basic group using neuropsychological tests, the mild cognitive disorders were detected in 50 (58.1 %) patients, and moderate cognitive disorders — in 36 (41.9 %). Neuropsychological pattern in patients with arrhythmias is represented by neurodynamic and regulatory disorders manifested in the deterioration of executive functions, auditory verbal me-mory, spatial gnosis, perception, concentration, decreased speed of psychomotor processes. Mild cognitive disorders were more common in patients with persistent (paroxysmal) atrial fibrillation (odds ratio (OR) 1.47, confidence interval (CI) 1.13–1.88, p = 0.036), moderate cognitive disorders — in those with permanent form of atrial fibrillation (OR 2.15, CI 1.45–3.32, p < 0.001) and with atrioventricular block degrees ІI–III (OR 2.62, CI 1.51–4.13, p < 0.001).
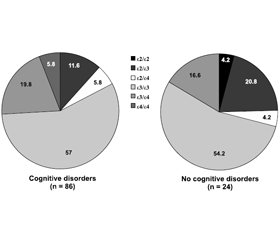
Analysis of the frequency of genotypes depending on the presence of cognitive disorders revealed a natural predominance of ε3/ε3 genotype, which occurred with a frequency of 57 % (in individuals with cognitive disorders) and 54.2 % (in patients without cognitive disorders) (p = 0.07). The least common was ε4/ε4 genotype, it was detected in 5.8 % of patients with cognitive disorders and was not found in people without cognitive impairment (p < 0.001). Among heterozygous genotypes, patients with cognitive disorders most often had ε3/ε4 genotype (19.8 %); its frequency in individuals without cognitive disorders was 16.6 % (p = 0.06). In patients with cognitive decline, ε2/ε3 genotype was detected in 11.6 % of cases, and those without cognitive disorders — in 20.8 % (p = 0.026) (Fig. 1).
/6.jpg)
Among individuals with cognitive disorders, 27 (31.4 %) carriers of at least one ε4 allele were identified. Other patients had ε2/ε2, ε2/ε3, ε3/ε3 genotypes, which according to the most literature data are not risk factors for neurodegenerative pathology.
People with mild cognitive disorders tended to accumulate genotypes ε2/ε3, ε3/ε3 and decrease genotypes ε2/ε4, ε3/ε4, which did not reach the level of statistical significance compared to the patients without cognitive impairment (p = 0.06) (Table 1). Among individuals with moderate cognitive disorders, there were no carriers of ε2/ε2 and ε2/ε3 genotypes, and the frequency of ε3/ε4, ε4/ε4 genotypes increased (p = 0.034).
Carrying the ε4 allele of the APOE gene at the trend level is associated with an increase in very-low-density lipoprotein cholesterol. Associations with the concentration of triglycerides and high-density lipoprotein cholesterol were not found. In patients with two ε4 alleles, plasma levels of total cholesterol and very-low-density lipoprotein cholesterol were significantly higher than in carriers of one allele and in the absence of the APOE4 genotype (p = 0.024 and p = 0.018, respectively). These results confirm the hypothesis of some researchers about the effect of APOE4 on the synthesis of very-low-density lipoprotein cholesterol [26].
Patients who were ε4 carriers by ε2/ε3/ε4 APOE polymorphism had higher C-reactive protein, interleukin-6 compared with non-carriers but no significant difference was found (p > 0.05 for both comparisons). The content of tumor necrosis factor α tended to increase in non-carriers of the ε4 allele compared to individuals with APOE4(+) (p = 0.06).
Patients with APOE4(+) compared to those with APOE4(–) had significantly higher indicators that characterized the condition of the lateral ventricles (p = 0.031). The width of the subarachnoid space at the level of the frontal pole differed significantly between the studied groups: 5.9 ± 0.5 mm vs. 4.2 ± 0.7 mm, respectively (p = 0.049). In the group of APOE4(+), the size of the third ventricle was 6.1 ± 0.5 mm, of APOE4(–), it was 5.7 ± 0.8 mm (p = 0.67). Patients with APOE4(+) were diagnosed with internal cerebral atrophy more than 1.6 times often (in 44.0 % of cases): mild — in 27.3 %, moderate — in 45.4 %, severe — in 27.3 %; in the APOE4(–) group, this indicator was 27.9 % (mild — in 58.8 %, moderate — in 41.2 %). When analyzing the morphometric characteristics, it can be stated that in patients of basic main group with the studied APOE genotype, cognitive disorders developed against the background of atrophic brain process.
APOE4 carriage had a greater negative impact on the mnestic sphere of cognitive activity (Table 2).
Differences in cognitive disorders depending on the pre-sence of the APOE4 genotype had various modalities and were associated with visual-spatial disorders (clock-drawing test) (r = –0.57, p < 0.001), memory disorders according to MMSE (r = –0.51, p < 0.001), 10 words (r = –0.54, p < 0.001) and 5 words tests (r = –0.47, p = 0.001), MDRS (r = 0.44, p = 0.003). Patients with APOE4 had more pronounced violations of regulatory functions, which was confirmed by a lower score on verbal associations (r = –0.51, p < 0.001), Trail Making Test, block B (r = –0.48, p = 0.001), Boston Naming Test (r = 0.46, p = 0.001).
Carriers of the APOE4(+) genotype had higher le-vels of depression and anxiety compared to those with the APOE4(–) genotype (OR 3.74, CI 1.72–7.38, p = 0.008). The presence of the ε4 allele contributes to the development of anxiety disorders and depression.
Carriage of the ε4 APOE allele is an additional factor that increases the risk of cognitive disorders in patients with arrhythmias, carriage of the ε2 allele can be considered a protective factor against cognitive disorders (Table 3).
Thus, a direct comparison of the results of neuropsychological research in patients with the APOE4(+) genotype revealed more pronounced cognitive decline. The APOE4(+) genotype is an unfavorable factor in the development of cognitive and affective disorders.
Conclusions
1. Carriage of the APOE4(+) genotype is associated with a greater rate of cognitive decline (OR 3.44, CI 1.94–5.15, p < 0.001), and carriage of the APOE4(–) genotype contributes to the preservation of cognitive functions (OR 0.73, CI 0.23–1.37, p < 0.001) in patients with cardiac arrhythmias.
2. Individuals with arrhythmias and risk factors for cognitive dysfunction should be genotyped with APOE, which will help identify groups at maximum risk of developing cognitive disorders and prevent them. If one or two ε4 alleles are detected, a control neuropsychological examination should be performed at least once every 3 months.
3. Further comprehensive study of the effect of APOE4 isoforms on the cognitive function of patients at risk of developing Alzheimer’s disease, in particular those with amnesic mild cognitive impairment against the background of arrhythmias, seems to be important for the search for new methods to predict the course of cognitive disorders and develop individualized approaches to their therapy.
Received 21.01.2022
Revised 02.02.2022
Accepted 07.02.2022
Список литературы
1. Бачинская Н.Ю. Лечение болезни Альцгеймера: современные возможности и перспективы. НейроNews: психоневрология и нейропсихиатрия. 2013. № 2(1). С. 1-7.
2. Мищенко Т.С. Когнитивные нарушения: актуальность, причины, диагностика, лечение, профилактика. Здоров’я України. Тематичний номер «Неврологія, психіатрія, психотерапія». 2017. № 1(40). С. 15-17.
3. Cunningham E.L., McGuinness B., Herron B., Passmore A.P. Dementia. Ulster Med. J. 2015. Vol. 84(2). P. 79-87. https://www.ums.ac.uk/umj084/084(2)079.pdf.
4. Prince M., Bryce R., Albanese E. The global prevalence of dementia: а systematic review and metaanalysis. Alzhei-mer’s & Dementia. 2013. Vol. 9(1). P. 63-75. doi: 10.1016/j.jalz.2012.11.007.
5. Головченко Ю.І., Горева Г.В., Слободін Т.М., Насонова Т.І., Гончар О.Ю. Клініко-нейропсихологічне співставлення когнітивного дефіциту із показниками системної та церебральної гемодинаміки при синдромі помірних когнітивних порушень. Зб. наук. праць співробіт. НМАПО імені П.Л. Шупика. 2015. № 24(2). С. 241-248.
6. Доценко Н.Я., Боев С.С., Шехунова И.А., Герасименко Л.В. Нарушение когнитивной функции у больных с артериальной гипертензией и дополнительными факторами риска, подходы к их коррекции. Therapia. 2016. № 10(113). С. 11-15.
7. Свиридова Н.К. Когнітивні та емоційно-особистісні порушення у хворих на гіпертензивну енцефалопатію. Стан мозкового кровообігу при артеріальній гіпертензії (науковий огляд та особисті спостереження). Міжнародний неврологічний журнал. 2016. № 1(79). С. 123-130.
8. Gottesman R.F., Schneider A.L., Albert M. Midlife hypertension and 20-year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014. Vol. 71(10). P. 1218-27. doi: 10.1001/jamaneurol.2014.1646.
9. Sierra C., Doménech M., Camafort M., Coca A. Hypertension and mild cognitive impairment. Current Hypertension Reports. 2012. Vol. 14(6). P. 548-55. doi: 10.1007/s11906-012-0315-2.
10. Lim Y.Y., Ellis K.A., Ames D. A-beta amyloid, cognition and APOE genotype in healthy older adults. Alzheimer’s & Dementia. 2013. Vol. 9. P. 538-45. doi: 10.1016/j.jalz.2012.07.004.
11. Mielke M.M., Leoutsakos J.-M., Tschanz J.T. Interaction between vascular factors and the APOE E4 allele in predicting rate of progression in Alzheimer’s dementia. J. Alzheimer’s Dis. 2011. Vol. 1. P. 127-34. doi: 10.3233/JAD-2011-110086.
12. Wang R., Fratiglioni L., Laukka E.J. Effects of vascular risk factors and APOE ε4 on white matter integrity and cognitive decline. Neurology. 2015. Vol. 84. P. 1128-35. doi: 10.1212/WNL.0000000000001379.
13. Лобзин В.Ю., Одинак М.М., Емелин А.Ю., Воробьев С.В. Особенности когнитивных нарушений, прогрессирования атрофии головного мозга и церебрального гипометаболизма у больных-носителей аллеля ε4 гена аполипопротеина Е. Тез. всеросс. науч.-практич. конф. «Давиденковские чтения». Санкт-Петербург: Изд-во «Человек и его здоровье», 2014. С. 136-137.
14. Peskind E.R., Li G., Shofer J. Age and apolipoprotein E4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch. Neurol. 2006. Vol. 63. P. 936-9. doi: 10.1001/archneur.63.7.936.
15. Deary I.J., Whiteman M.C., Pattie A. Cognitive change and the APOE e4 allele. Nature. 2002. Vol. 6901. P. 932. doi: 10.1038/418932a.
16. Dik M.G.C., Jonker M.D., Comijs H.C. Memory complaints and APOE-4 accelerate cognitive decline in cognitively normal elderly. Neurology. 2001. Vol. 12. P. 2217-22. doi: 10.1212/wnl.57.12.2217.
17. Geda Y.E., Knopman D.S., Mrazek D.A. Depression, apolipoprotein E genotype, and the incidence of mild cognitive impairment: a prospective cohort study. Arch. Neurol. 2006. Vol. 63. P. 435-40. doi: 10.1001/archneur.63.3.435.
18. Мілевська-Вовчук Л.С. Порівняльна характеристика скринінгових шкал для виявлення когнітивних порушень. Міжнародний неврологічний журнал. 2015. № 8(78). С. 41-44.
19. Dubois B., Slachevsky A., Litvan I., Pillon B. The FAB: a frontal assessment battery at bedside. Neurology. 2000. Vol. 55. P. 1621-6.
20. Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975. Vol. 12. P. 189-98.
21. Белова А.Н. Шкалы, тесты и опросники в неврологии и нейрохирургии: Руководство для врачей и научных работников. Москва, 2004. 432 с.
22. Блейхер В.М., Крук И.В., Боков С.Н. Клиническая патопсихология. Руководство для врачей и клинических психологов. Москва-Воронеж: НПО «МОДЭК», 2002. С. 68-102.
23. Евтушенко О.С., Яновская Н.В., Сухоносова О.Ю. Шкалы в общей и детской неврологии: научно-практическое и методическое пособие. Киев: Заславский А.Ю., 2015. 104 с.
24. Лурия А.Р. Основы нейропсихологии. Москва: Издательство МГУ, 1973. 217 с.
25. Мищенко Т.С., Шестопалова Л.Ф., Трищинская М.А. Клинические шкалы и психодиагностические тесты в диагностике сосудистых заболеваний головного мозга (методические рекомендации). Харьков, 2008. 36 с.
26. Younus S., Rodgers G. Biomarkers associated with cardiometabolic risk in obesity. Am. Heart Hosp. J. 2011. Vol. 9(1). P. 28-32. doi: 10.15420/ahhj.2011.9.1.28.

/6.jpg)
/7.jpg)